Novel structure of the N-terminal helical domain of BibA, a Group B Streptococcus immunogenic bacterial adhesin
Kartik Manne, Debasish Chattopadhyay, Vaibhav Agarwal, Anna M. Blom, Baldeep Khare, Srinivas Chakravarthy, Chungyu Chang, Hung Ton-That and Sthanam V. L. Narayana
[1]
Molecular Tour
This publication describes a high–resolution X-ray crystallographic structure of the Group B Streptococcus BibA N-terminal fragment (BibA126-398), and a low-resolution structure of the full-length N-terminal domain (BibA34-400) determined using small angle X-ray scattering (SAXS). The association of the BibA N-terminal domain was localized to the C4BP α-chain.
shown as a rainbow model with color changing gradually from the N-terminus (blue) to the C-terminus (red). The antiparallel three-helix-bundle-motif repeats are labeled from the N-terminus to the C-terminus as MR1–MR4, respectively.
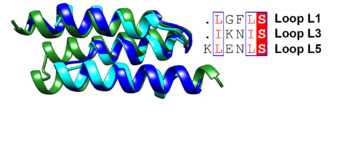
Superposition of the antiparallel three-helix-bundle-motif repeats MR1 (dark blue), MR2 (cyan) and MR3 (forest green). Similar residues such as Leu and Ile are boxed and shown in a red font and the conserved serine residue is highlighted in red.
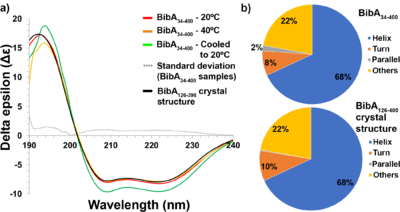
(a) Circular dichroism spectra of freshly prepared and intact BibA34–400 sample at 20°C (red), at 40°C (orange) and denatured at 80°C followed by cooling to 20°C (green), the standard deviation error between the BibA34–400 samples (gray dotted line) and the spectrum generated from the BibA126–398 crystal structure (black). Two negative peaks at 208 and 222 nm typical of a α-helical secondary structure were observed for the BibA34–400 sample. (b) Estimated secondary-structure content (%) of BibA34–400 sample in solution (top) and the spectrum generated from the BibA126–398 crystal structure (bottom).
(a) Pair distance distribution [P(r)] function of intact BibA34–400 protein. (b) Comparison of the experimental scattering profile (in blue) for BibA34–400 with profiles from a theoretical model (FoXS; green) derived from the proposed BibA34–400 model. (c) Fit of the crystal structure of BibA126–398 (cyan) and the proposed BibA34–400 (magenta) into the ab initio model of BibA34–400 calculated with
DAMMIF.
References
- ↑ Manne K, Chattopadhyay D, Agarwal V, Blom AM, Khare B, Chakravarthy S, Chang C, Ton-That H, Narayana SVL. Novel structure of the N-terminal helical domain of BibA, a group B streptococcus immunogenic bacterial adhesin. Acta Crystallogr D Struct Biol. 2020 Aug 1;76(Pt 8):759-770. doi:, 10.1107/S2059798320008116. Epub 2020 Jul 27. PMID:32744258 doi:http://dx.doi.org/10.1107/S2059798320008116




