We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Simvastatin Synthase
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
| + | __TOC__ | ||
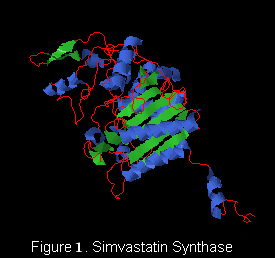
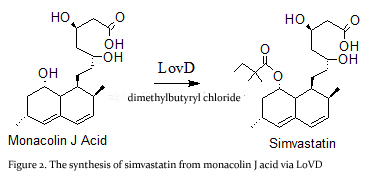
'''Simvastatin synthase''' or '''transesterase''' (LovD) is a 46 kDa acyltransferase found in the lovastatin biosynthetic pathway and catalyzes the final step of [[Lovastatin]] biosynthesis<ref name="paper4">PMID:17113998</ref>. Pictured here is the generated double mutant C40A/C60N (G0), from wild type LovD (Figure 1).This enzyme is isolated from the natural product biosynthetic pathways of [http://en.wikipedia.org/wiki/Aspergillus_terreus ''Aspergillus terreus''], specifically the polyketide biosynthetic pathway. Simvastatin Synthase converts the inactive monacolin J acid (MJA) by dimethylbutyryl chloride to yield the protected form of simvastatin (Figure 2), which subsequently undergoes lactonization to yield [[Simvastatin]]<ref name="paper5">PMID:19875080</ref>. | '''Simvastatin synthase''' or '''transesterase''' (LovD) is a 46 kDa acyltransferase found in the lovastatin biosynthetic pathway and catalyzes the final step of [[Lovastatin]] biosynthesis<ref name="paper4">PMID:17113998</ref>. Pictured here is the generated double mutant C40A/C60N (G0), from wild type LovD (Figure 1).This enzyme is isolated from the natural product biosynthetic pathways of [http://en.wikipedia.org/wiki/Aspergillus_terreus ''Aspergillus terreus''], specifically the polyketide biosynthetic pathway. Simvastatin Synthase converts the inactive monacolin J acid (MJA) by dimethylbutyryl chloride to yield the protected form of simvastatin (Figure 2), which subsequently undergoes lactonization to yield [[Simvastatin]]<ref name="paper5">PMID:19875080</ref>. | ||
Revision as of 07:51, 13 August 2024
| |||||||||||
3D structures of simvastatin synthase
Updated on 13-August-2024
3hl9, 3hlb, 3hlc, 4lcl, 4lcm – AtLovD (mutant) – Aspergillus terreus
3hld, 3hle – AtLovD (mutant) + monacolin J acid
3hlf – AtLovD (mutant) + simvastatin
3hlg – AtLovD (mutant) + lovastatin
References
- ↑ 1.0 1.1 1.2 Xie X, Watanabe K, Wojcicki WA, Wang CC, Tang Y. Biosynthesis of lovastatin analogs with a broadly specific acyltransferase. Chem Biol. 2006 Nov;13(11):1161-9. PMID:17113998 doi:10.1016/j.chembiol.2006.09.008
- ↑ Gao X, Xie X, Pashkov I, Sawaya MR, Laidman J, Zhang W, Cacho R, Yeates TO, Tang Y. Directed evolution and structural characterization of a simvastatin synthase. Chem Biol. 2009 Oct 30;16(10):1064-74. PMID:19875080 doi:10.1016/j.chembiol.2009.09.017
- ↑ 3.0 3.1 3.2 3.3 Xie X, Tang Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysis. Appl Environ Microbiol. 2007 Apr;73(7):2054-60. Epub 2007 Feb 2. PMID:17277201 doi:10.1128/AEM.02820-06
- ↑ Kennedy J, Auclair K, Kendrew SG, Park C, Vederas JC, Hutchinson CR. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science. 1999 May 21;284(5418):1368-72. PMID:10334994
- ↑ 5.0 5.1 Xie X, Pashkov I, Gao X, Guerrero JL, Yeates TO, Tang Y. Rational improvement of simvastatin synthase solubility in Escherichia coli leads to higher whole-cell biocatalytic activity. Biotechnol Bioeng. 2009 Jan 1;102(1):20-8. PMID:18988191 doi:10.1002/bit.22028
- ↑ Wagner UG, Petersen EI, Schwab H, Kratky C. EstB from Burkholderia gladioli: a novel esterase with a beta-lactamase fold reveals steric factors to discriminate between esterolytic and beta-lactam cleaving activity. Protein Sci. 2002 Mar;11(3):467-78. PMID:11847270
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Eric Ginter, David Canner, Alexander Berchansky