The product of the human tumor susceptibility gene 101 (Tsg101) is a component of the endocytic sorting complex required for transport (ESCRT-1 complex), which recognizes ubiquitinated protein cargo and facilitates cargo delivery to sub-cellular compartments for degradation. Ironically, Tsg101 also is required for release of the human immunodeficiency virus (HIV) from cells.
Background and History
The Human Immunodefiency Virus (HIV) is the agent causing acquired immunodefiency syndrome (AIDS) in which the immune system of a human begins to deteriorate, leading to life threatening infections. HIV is a retrovirus that uses its host's cellular machinery to replicate. The Tumor susceptibility gene 101 (Tsg101), is a human gene that codes for a cellular protein, plays an important role in the pathogenesis of HIV, and belongs to the ubiquitin-conjugating enzyme family, the ubiquitin E2 variant (UEV) subfamily.
In normal functioning cells, Tsg101, as a subunit of the ESCRT-1 (Endosomal Sorting Complex Required for Transport), promotes membrane alterations inside the cell that result in the formation of compartments know as multivesicular bodies (MVB image) in which proteins that have been marked for transport to the lysosome, through ubiquination, are sorted. Normally, a specific tetrapeptide PSAP binding motif on the endosomal protein Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) will bind to the specific P(S/T)AP binding pocket on the Tsg101 and deliver the protein cargo to endosomes that eventually become or fuse with lysosomes. If HIV is present in a cell, a major structural protein of the virus, Gag, recruits the Tsg101 protein using its own PTAP motif, which mimics the Hrs and binds to the binding pocket of the Tsg101 to gain access to the downstream machinery and to mediate viral budding as an alternative to degradation. The Gag gene encodes the genetic information for the structural proteins of retroviruses. PTAP and PSAP motifs are able to bind Tsg101 equally.
Structural and Functional Properties of the Tsg101
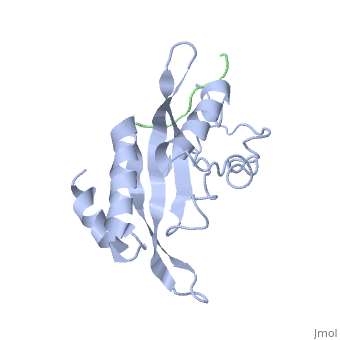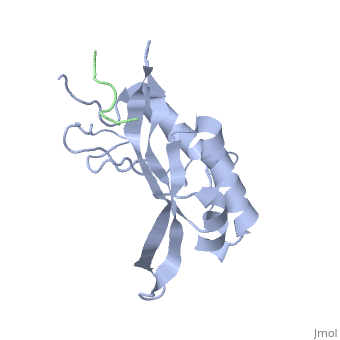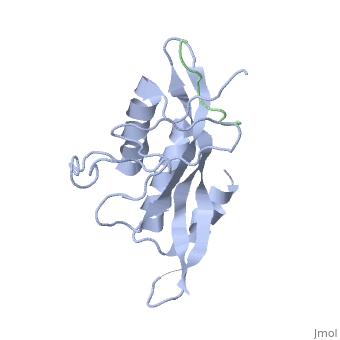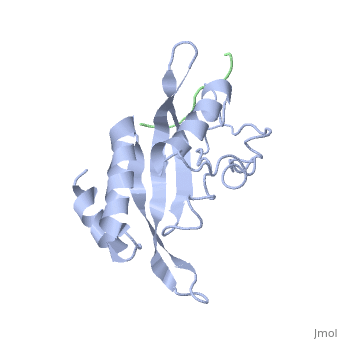
The features the wild type version of the , amino acid residues Met 1 through Pro 145 and the protein, amino acid residues Pro 205 through Glu 213. Focusing on the Tsg101 UEV domain of this PDB, important features of this protein are observed that may be directly involved in the normal trafficking or viral budding processes. The Tsg101 protein contains a region designated as the UEV domain due to its overall alpha/beta/alpha/ loop configuration that is similar to other E2 ubiquitin ligases, but it does not have the C-terminal helices found in many other E2 enzymes.
The Tsg101 UEV domain features the represented by amino acid residues: Thr 58; Val 61; Tyr 63; Tyr 68; Asn 69; Ile 70; Pro 71; Thr 92; Met 95; Pro 139; Val 141; Phe 142; Ser 143 and Arg 144. This binding pocket, which under normal conditions binds the protein Hrs, can also bind the HIV-1 Gag protein.
Another important feature on the Tsg101 that may be involved in the normal trafficking or viral budding process is the ubiquitin binding pocket. The of the Tsg101 UEV domain has eight specific residues that are involved: residues 43 through 49; and residue 88. The flanking or end residues are Valine 43 and Phenylalanine 88. These residues are highlighted because they mark the beginning and end of the ubiquitin binding pocket. The binding of ubiquitin is thought to strengthen the Tsg101 - HIV-1 Gag interaction and it is speculated that it assists the complex into the trafficking lane and onto the membrane for budding. However, polyubiquitination (the binding of multiple ubiquitin, usually more than four) to any protein, including the Tsg101, will mark it for degradation.
A third structural feature of the Tsg101 UEV domain that may be indirectly involved in the overall trafficking and viral budding is amino acid residues occupying the C-terminal end of the UEV domain. Mutations in this area are discussed below.
Mutations - PTAP Binding Pocket, Ubiquitin Binding Pocket, C-terminal UEV Domain
Identification of mutations of various residues that inhibit HIV-1 Gag binding can provide important information and avenues for future research aimed at the discovery of new anti-viral agents. featured at the top of this page represents the wild type version of Tsg101 UEV domain and it highlights residues that are directly or indirectly related to the P(S/T)AP and ubiquitin binding pockets and/or residues found by mutational analysis, which are important in the interaction. The residues fall into four groups: specific residues found within or around the P(S/T)AP binding pocket - , , (green); residues in the ubiquitin binding pocket, with the focus on the two end or flanking residues of the pocket - , (cyan); residues unique to Tsg101 that are not conserved in related E2 enzymes - , , (orange); and residues conserved in Tsg101 and related E2 enzymes - (yellow).
The three residues featured in the P(S/T)AP binding pocket exhibit potential areas where mutations can occur, which may eliminate or reduce the HIV-1 Gag protein binding to the Tsg101
P(S/T)AP binding pocket. The two residues in the ubiquitin binding pocket feature potential sites where mutations can occur that inhibit the binding of a ubiquitin-modified peptide derived from the PTAP-containing region of Gag. The C-terminal region of the UEV domain where the mutation of certain amino acid residues, which are not directly involved in the P(S/T)AP binding pocket result in the elimination or reduction of binding to the Gag protein. A mutation of W117, which is conserved in all E2 enzymes, to A117 eliminates binding entirely. Mutations of Y110, Y113 and K118, which are unique to the Tsg101, to W110, V113 and A118 reduce binding by 31%, 33% and 50% respectively (VerPlank et al. 2001). These reduction and eliminations of binding may be caused by overall conformational changes of the P(S/T)AP binding pocket or the ubiquitin binding pocket that result from the various steric effects or different hydrogen bonding resulting from the mutated amino acids on the C-terminal UEV end of the Tsg101.
Future Research
There are a number of sites on Tsg101 that are involved in normal trafficking or viral budding functions (VerPlank et al. 2001, Pornillos et al. 2002). The binding of HIV-1 Gag to the
P(S/T)AP binding pocket works in coordination with the binding of the ubiquitin. Perhaps, when HIV-1 Gag is bound in the pocket, Gag containing more than one ubiquitin molecule cannot bind in the P(S/T)AP binding pocket or more than one ubiquitin molecule cannot be added to Gag, thereby preventing Gag from being sorted to the normal degradation pathway.
BIAcore biosensor experiments conducted by Pornillos, et al. 2003, showed that the binding affinities of the P(S/T)AP motifs in wild type and mutated HIV-1 Gag and Hrs exhibit variation. Binding affinity was greatest for the sequence in HIV-1 Gag as compared with Hrs and the mutated HIV-1 Gag. The binding affinities were shown to be directly attributed to Tsg101-Gag binding because when the proline was mutated to leucine, no binding was detected. Specific sequences flanking the central P(S/T)AP motif appeared to affect the absolute binding affinities between Tsg101 and Hrs. These data suggest that when specific residues located in the P(S/T)AP binding pocket of Tsg101 are mutated, there will be a reduction in the binding affinity not only for the HIV-1 Gag, but also for other cellular proteins such as Hrs. In addition to these binding affinity data, a specific 8-member cyclic peptide, which was selected for in an experiment conducted by Tavassoli, et al. 2008, was shown to decrease the interaction of both Gag-Tsg101 and Hrs-Tsg101. This further supports the importance of understanding the interaction of Gag-Tsg101 in the P(S/T)AP binding site and flanking residues sites, and illustrates the possibility of inhibiting specific protein-protein interactions which may lead to viral budding.
Tsg101 UEV domain residues Y110, Y113, and K118 are unique to Tsg101, while W117 is conserved in E2 enzymes and Tsg101. Mutations in all of these specific sites appear to cause a significant decrease in Gag interaction with the P(S/T)AP binding pocket. In the case of the unique residues, the alteration may have altered sites critical for the structure of the catalytically unresponsive active site in Tsg101, which as noted, is a variant form of the E2 enzyme. In the case of the conserved W117 residue, mutations may have disrupted a site important for overall protein folding or stability. Most significant is the fact that these residues affect binding, which suggests that anti-viral strategies currently being developed against SH3 and WW domain protein-binding motifs might be employed. As in Tsg101, the critical factors determining binding are regions containing aromatic residues (Y110, Y113 and W117 in Tsg101), which are flanked by charged residues (K108 and K118 in Tsg101). As mentioned above, this decrease may be attributed to significant conformational changes within the Tsg101 protein that occurs when different steric effects are experienced either through mutations or other binding peptides.
Polymers of N-substituted glycine (NSG) residues have recently emerged as an important class of peptide inhibitors that effectively can substitute for proline in WW and SH3 domain-binding proteins. In the Tsg101 UEV, the Ala 9 - Pro 10 binding pocket resembles the x-proline pockets of WW and SH3 domains. SH3 domains are about 50 to 70 amino acids long and are often present in proteins responsible for eukaryotic signal transduction and are found in the cytoskeleton. These domains recognize proline rich ligands with a PxxP motif (where x is any amino acid and P is proline). WW domains are the smallest protein-binding modules and are made up of approximately 40 amino acids. The name refers to two tryptophan residues spaced approximately 20 to 22 amino acids apart, which also recognize proline-rich ligands. While WW domains resemble SH3 domains functionally by binding to proline rich ligands, WW domains bind to PPxY motifs (where Y is tyrosine). The key proline residues in ligands that bind to WW and SH3 domains take on polyproline helical conformations like PTAP and bind with the proline ring wedged between two perpendicularly situated aromatic rings on the domains. The positions of the key prolines and the two aromatic rings are conserved across the Tsg101 UEV P(S/T)AP, WW PPxY and SH3 PxxP complexes. This is significant because in order for viral budding to occur, Tsg101 has to bind to the PTAP region of the Gag and N-substituted 'peptoid' inhibitors have been identified to successfully compete with the binding of proline rich ligands to WW and SH3 domains. The similarities between Tsg101 and SH3 domains in their recognition of key proline residues suggest that the replacement of proline residues with NSG constructs may eventually lead to the preparation of PTAP-based Tsg101 binding inhibitors that can potentially prevent viral budding.