We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Leanne Price/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
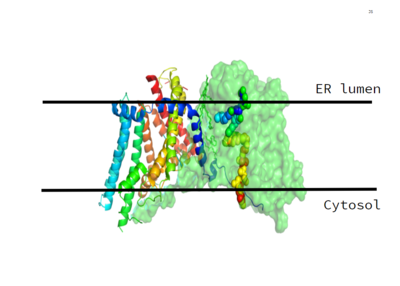
The active site of DGAT is located within the membrane, with the catalytic histidine residue (<scene name='87/877601/His415/2'>His415</scene>-represented in white) buried inside the central cavity. This central cavity serves as the catalytic site. The acyl-acceptor lipid substrates access the active site through the lateral gate within the membrane. The active site also contains <scene name='87/877601/His415/1'>His415</scene> (represented in white) and several nearby polar residues (including Asn378, Gln437, and Gln465) whose side chains are oriented towards the cavity center. These residues interact and create a hydrophilic channel within the active site. The His415 residue is also likely involved in catalysis, making it increasingly significant. | The active site of DGAT is located within the membrane, with the catalytic histidine residue (<scene name='87/877601/His415/2'>His415</scene>-represented in white) buried inside the central cavity. This central cavity serves as the catalytic site. The acyl-acceptor lipid substrates access the active site through the lateral gate within the membrane. The active site also contains <scene name='87/877601/His415/1'>His415</scene> (represented in white) and several nearby polar residues (including Asn378, Gln437, and Gln465) whose side chains are oriented towards the cavity center. These residues interact and create a hydrophilic channel within the active site. The His415 residue is also likely involved in catalysis, making it increasingly significant. | ||
| + | ==Mechanism== | ||
| + | |||
| + | In the DGAT mechanism, the diglyceride serves as the nucleophile. While the acyl group of the CoA enzyme serves as the electrophile. The lone pair on the last hydroxyl group present on the glycerol of the diglyceride attacks the thioester bond of the acyl-CoA enzyme. This attack breaks the sulfur-carbon bond, a weak bond that is easily breakable. This allows the acyl group of the acyl-CoA enzyme to attach to the diglyceride, creating a triglyceride. While the CoA group then serves as the leaving group. | ||
| + | |||
| + | ===Leaving Group=== | ||
| + | |||
| + | As previously mentioned, the Acyl-CoA molecule serves as the leaving group in the DGAT mechanism. This acyl-CoA molecule occupies the cytosolic tunnel, which has a bent architecture. The CoA moiety is at the cytosolic face, while the acyl chain extends through the center towards the endoplasmic reticulum lumen. The distal end of the acyl chain oleoyl-CoA interacts with DGAT deep within the hydrophobic channel of the active site. As the acyl-CoA binds to DGAT, small conformational changes are seen in the active site region. Although the reason for these conformational changes has yet to be discovered, it has been proposed that they prepare the enzyme for catalysis, making them very significant. | ||
== Relevance == | == Relevance == | ||
Revision as of 23:25, 17 March 2021
DGAT Human
| |||||||||||
References
- ↑ Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Justin Smith
- Eloi Bigirimana
- Leanne Price