User:Madison Unger/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=ACAT/SOAT= | =ACAT/SOAT= | ||
<StructureSection load='6l47' size='340' frame='true' side='right' caption='ACAT/SOAT 6l47' scene='87/877507/His460/1'> | <StructureSection load='6l47' size='340' frame='true' side='right' caption='ACAT/SOAT 6l47' scene='87/877507/His460/1'> | ||
| - | This is a default text for your page '''Madison Unger/Sandbox 1'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| - | |||
== Introduction == | == Introduction == | ||
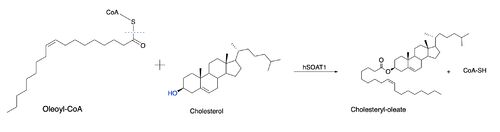
[[Image:overallstructureacat.png|400px|left|thumb|Figure 1.Tetrameric dimer of dimer for ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage. [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | [[Image:overallstructureacat.png|400px|left|thumb|Figure 1.Tetrameric dimer of dimer for ACAT]] Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage. [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) and ghrelin O-acyltransferase ([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). | ||
| Line 30: | Line 27: | ||
The mechanism of ACAT is essential for cholesterol storage and cholesterol transfer through the plasma because cholesteryl ester is the primary form of cholesterol used for these events. Additionally, ACAT can use a variety of different sterol molecules besides cholesterol as substrates and activators. Because of its biological importance, ACAT has been linked to [http://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis], [http://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer’s disease], and cancer as a potential drug target for treatment of these diseases. Various studies have looked into ACAT inhibition and how that inhibition treats or prevents certain diseases, such as reducing the size and metastasis of certain tumors and reducing the formation of plaques in atherosclerosis. ACAT is an important target for these diseases due to its functional relevance in cholesterol metabolism. | The mechanism of ACAT is essential for cholesterol storage and cholesterol transfer through the plasma because cholesteryl ester is the primary form of cholesterol used for these events. Additionally, ACAT can use a variety of different sterol molecules besides cholesterol as substrates and activators. Because of its biological importance, ACAT has been linked to [http://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis], [http://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer’s disease], and cancer as a potential drug target for treatment of these diseases. Various studies have looked into ACAT inhibition and how that inhibition treats or prevents certain diseases, such as reducing the size and metastasis of certain tumors and reducing the formation of plaques in atherosclerosis. ACAT is an important target for these diseases due to its functional relevance in cholesterol metabolism. | ||
| - | == Structural highlights == | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="<scene name='87/877507/Zoomed_in_on_his_arg_scene/1'>Zoomed in structure</scene>">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.<ref name="Ransey">PMID:28504306</ref> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 20:11, 29 March 2021
ACAT/SOAT
| |||||||||||
References
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Rogers MA, Liu J, Song BL, Li BL, Chang CC, Chang TY. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J Steroid Biochem Mol Biol. 2015 Jul;151:102-7. doi: 10.1016/j.jsbmb.2014.09.008., Epub 2014 Sep 12. PMID:25218443 doi:http://dx.doi.org/10.1016/j.jsbmb.2014.09.008
Student Contributors
- Leah Goehring
- Gabby Smith
- Anna Campbell