We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Allison Welz/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Function == | == Function == | ||
| - | === Mechanism === | + | ====Mechanism==== |
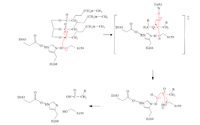
| + | [[Image:MechFinalAllison.png|200 px|]] | ||
| + | # The triglyceride binds to LPL’s lipid-binding region in an open lid conformation. | ||
| + | # The oxygen on S159 is made more [https://en.wikipedia.org/wiki/Nucleophile nucleophilic]. This happens via [https://en.wikipedia.org/wiki/Histidine histidine] hydrogen bonding with the hydrogen on S159’s alcohol group. | ||
| + | # The nucleophilic oxygen attacks the [https://en.wikipedia.org/wiki/Carbonyl_group carbonyl carbon] of one of the fatty acid chains. | ||
| + | # This pushes electrons up onto the carbonyl oxygen, creating a [http://www.chem.ucla.edu/~harding/IGOC/T/tetrahedral_intermediate.html tetrahedral intermediate]. This is the oxyanion hole which is stabilized by main chain nitrogen atoms of W82 and L160. | ||
| + | # One of the lone pairs of the oxygen (in the oxyanion hole) creates a double bond carbon. | ||
| + | # The oxygen-carbon bond between the single fatty acid chain and the [https://en.wikipedia.org/wiki/Diglyceride diglyceride] is cleaved. | ||
| + | # H268 hydrogen bonds water, making the oxygen a better nucleophile. Water attacks the carbonyl carbon. | ||
| + | # The [https://en.wikipedia.org/wiki/Carboxylic_acid carboxylic acid] is formed and the S159 bond is cleaved and re-protonated via H268. | ||
| + | # The active site is now back in its original state. | ||
== Disease == | == Disease == | ||
Revision as of 21:08, 26 April 2021
Contents |
LPL with GPIHBP-1, Drosophilia S2
| |||||||||||
M404R Mutation
| |||||||||||
Relevance
Structural highlights
</StructureSection>
Student Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>