User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
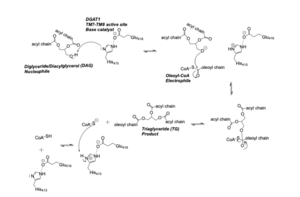
DGAT synthesizes triacylglycerides from diacylglycerol (DAG) and fatty acyl-CoA. The catalytic mechanism for triacylglyceride synthesis by DGAT is shown in Figure 1. | DGAT synthesizes triacylglycerides from diacylglycerol (DAG) and fatty acyl-CoA. The catalytic mechanism for triacylglyceride synthesis by DGAT is shown in Figure 1. | ||
| - | + | ||
== Structure == | == Structure == | ||
| + | |||
=== Dimer Interface === | === Dimer Interface === | ||
| + | <scene name='87/877512/Dimer_interface/5'>H bonding at interface</scene> | ||
| + | |||
=== Active Site === | === Active Site === | ||
| + | |||
=== DAG Binding === | === DAG Binding === | ||
| + | |||
=== Acyl-CoA Binding === | === Acyl-CoA Binding === | ||
| - | + | ||
| + | |||
== Disease == | == Disease == | ||
Revision as of 16:14, 4 April 2021
Diacylglycerol acyltransferase, DGAT
| |||||||||||
References
- ↑ 1.0 1.1 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa