User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
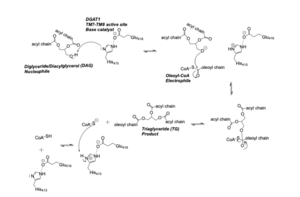
| - | Diacylglycerol acyltransferase (DGAT) is a membrane protein that synthesizes triacylglycerides from its two substrates diacylglycerol (DAG) and fatty acyl-CoA for dietary fat absorption and fat storage. DGAT can be found expressed in the small intestine’s epithelial cells, in the liver where it synthesizes fat for storage, and in the female mammary glands where it produces fat for milk <ref name="Wang">PMID: 32433610</ref>. DGAT is a member of the membrane-bound O- acyltransferases (MBOAT) family <ref name="Sui">PMID: 32433611</ref>. All of the enzymes within this family are transmembrane enzymes that acylate lipids or proteins. Additionally, MBOAT enzymes have a conserved MBOAT core, a channel-like region that acts as the enzyme’s active site. Another feature of note within this MBOAT core is the conserved catalytic Histidine. | + | Diacylglycerol acyltransferase (DGAT) is a membrane protein that synthesizes triacylglycerides from its two substrates diacylglycerol (DAG) and fatty acyl-CoA for dietary fat absorption and fat storage. DGAT can be found expressed in the small intestine’s epithelial cells, in the liver where it synthesizes fat for storage, and in the female mammary glands where it produces fat for milk <ref name="Wang">PMID: 32433610</ref>. DGAT is a member of the membrane-bound O- acyltransferases (MBOAT) family <ref name="Sui">PMID: 32433611</ref>. All of the enzymes within this family are transmembrane enzymes that acylate lipids or proteins. Additionally, MBOAT enzymes have a conserved MBOAT core, a channel-like region that acts as the enzyme’s active site. Another feature of note within this MBOAT core is the conserved catalytic Histidine. DGAT is a dimer that has two identical subunits. Each of the individual subunits contains an MBOAT core that acts as its active site. Each subunit also contains nine transmembrane alpha-helices (TM), 2 intracellular loops (IL), and one ER luminal loop (EL). TM2-9, IL1, and IL2 form the structure of the MBOAT core active site. |
| - | + | ||
== Function == | == Function == | ||
| - | |||
DGAT synthesizes triacylglycerides from diacylglycerol (DAG) and fatty acyl-CoA. | DGAT synthesizes triacylglycerides from diacylglycerol (DAG) and fatty acyl-CoA. | ||
| Line 17: | Line 15: | ||
=== Dimer Interface === | === Dimer Interface === | ||
| - | DGAT is a dimer that has two identical subunits with 9 transmembrane alpha helices (TM), 2 intracellular loops (IL), and one ER luminal loop (EL). The dimer is held together at the dimer interface. Both <scene name='87/877512/Dimer_interface/5'> | + | DGAT is a dimer that has two identical subunits with 9 transmembrane alpha helices (TM), 2 intracellular loops (IL), and one ER luminal loop (EL). The dimer is held together at the dimer interface. Both <scene name='87/877512/Dimer_interface/5'>Hydrogen bonding</scene> and hydrophobic interactions between the residues of the TM1 and EL1 regions of both subunits act to hold the subunits of the dimer together. |
Revision as of 02:34, 5 April 2021
Diacylglycerol acyltransferase, DGAT
| |||||||||||
References
- ↑ 1.0 1.1 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa