User:Hannah Wright/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
===GPIHBP1=== | ===GPIHBP1=== | ||
====Calcium Ion Coordination==== | ====Calcium Ion Coordination==== | ||
| - | + | ===Active Site=== | |
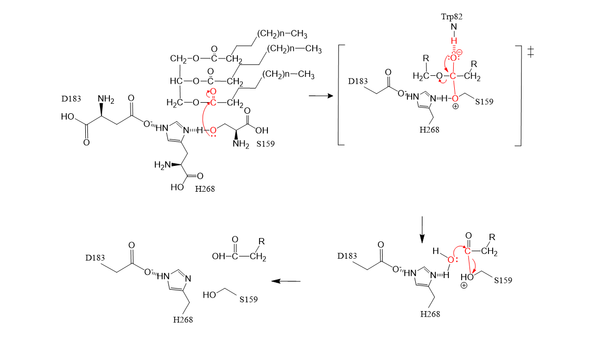
The <scene name='87/878236/Active_site_possibly_done/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_res_w_ligand/1'>hydrophobic tunnel</scene>, including residues: W82, V84, W113, Y121, Y158, L160, A185, P187, F212, I221, F239, V260, V264 and K 265, outline the general structure of the active site and provides considerable stability of the active site. The <scene name='87/878236/Active_site_lid_region_done/1'>lid region</scene> is also an important component of the active site, spanding from residues 243-266, that is vital for the recognition of substrates. The <scene name='87/877636/Activesite_oxyanionhole/2'>oxyanion hole</scene>, which is a small portion of the hydrophobic tunnel consisting of residues L160, and W82, aids in the overall stability of the active site and the transition state of substrates catalyzed by the <scene name='87/877603/Catalytic_triad/1'>catalytic triad</scene> (residues H268, S159, and D183). | The <scene name='87/878236/Active_site_possibly_done/2'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_hphob_res_w_ligand/1'>hydrophobic tunnel</scene>, including residues: W82, V84, W113, Y121, Y158, L160, A185, P187, F212, I221, F239, V260, V264 and K 265, outline the general structure of the active site and provides considerable stability of the active site. The <scene name='87/878236/Active_site_lid_region_done/1'>lid region</scene> is also an important component of the active site, spanding from residues 243-266, that is vital for the recognition of substrates. The <scene name='87/877636/Activesite_oxyanionhole/2'>oxyanion hole</scene>, which is a small portion of the hydrophobic tunnel consisting of residues L160, and W82, aids in the overall stability of the active site and the transition state of substrates catalyzed by the <scene name='87/877603/Catalytic_triad/1'>catalytic triad</scene> (residues H268, S159, and D183). | ||
Revision as of 19:17, 6 April 2021
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
Student/Contributors
- Ashrey Burely
- Allison Welz
- Hannah Wright