We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Leanne Price/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
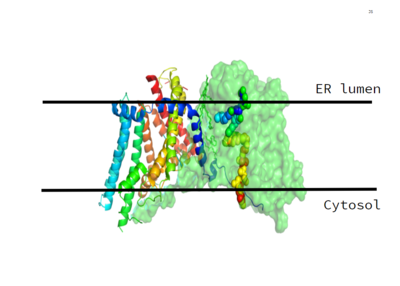
[[Image:Image_1.PNG|400 px|left|thumb|Figure 1. This image shows the location of the DGAT protein within the Endoplasmic Reticulum Membrane]] | [[Image:Image_1.PNG|400 px|left|thumb|Figure 1. This image shows the location of the DGAT protein within the Endoplasmic Reticulum Membrane]] | ||
| - | DGAT, or Diacylglycerol Acyltransferase is a polytopic endoplasmic reticulum membrane protein embedded within the membrane of the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum ER]. DGAT is highly expressed in epithelial cells of the small intenstine of homo sapiens. It can also be found in the liver, where it helps synthesize fats for storage, and the female mammary glands, where it produces fat in the milk. | + | DGAT, or Diacylglycerol Acyltransferase, is a polytopic endoplasmic reticulum membrane protein embedded within the membrane of the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum ER]. DGAT is highly expressed in epithelial cells of the small intenstine of homo sapiens. It can also be found in the liver, where it helps synthesize fats for storage, and the female mammary glands, where it produces fat in the milk. |
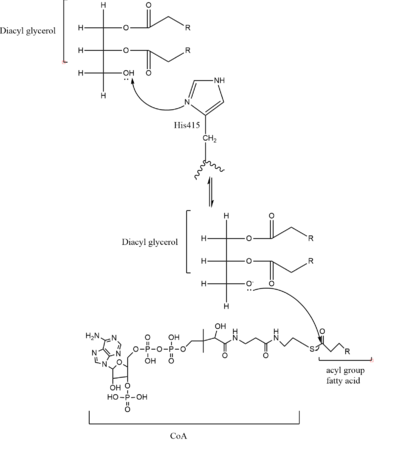
| - | DGAT was originally discovered by its homology to [https://en.wikipedia.org/wiki/Sterol_O-acyltransferase Acyl-CoA cholesterol acyltransferases (ACAT) 1 and 2]. The structure, catalytic mechanism of diacylglycerol acyltransferase, and how DGAT interacts with CoA was discovered using a [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-EM]. The Cryo-EM map revealed that DGAT forms a dimer, with each subunit containing nine transmembrane helices. The N and C terminals of each helix are located on the cytosolic and luminal sides of the endoplasmic reticulum membrane respectively. | + | DGAT was originally discovered by its homology to [https://en.wikipedia.org/wiki/Sterol_O-acyltransferase Acyl-CoA cholesterol acyltransferases (ACAT) 1 and 2]. The structure, catalytic mechanism of diacylglycerol acyltransferase, and how DGAT interacts with CoA was discovered using a [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-EM]. The Cryo-EM map revealed that DGAT forms a dimer, with each subunit containing nine transmembrane helices. The N and C terminals of each helix are located on the cytosolic and luminal sides of the endoplasmic reticulum membrane respectively (Figure 1). |
Revision as of 19:50, 6 April 2021
Diacylglycerol Acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 https://www.proteinatlas.org/ENSG00000185000-DGAT1/pathology
- ↑ Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Justin Smith
- Eloi Bigirimana
- Leanne Price