We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Leanne Price/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
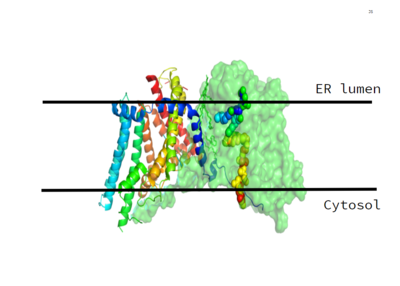
The cleavage site for oleoyl-CoA is within a short distance of a lipid acceptor. This revelation was made after a strong, lipid-like density in the central cavity in the cryo-EM data. Hydrophobic residues line this region and form a channel surrounding the lipid-like density. The channel itself has a bent, hydrophobic pathway that allows the binding of hydrophobic molecules. The bent architecture of this tunnel is likely how DGAT distinguishes acyl acceptors from other molecules, such as cholesterol. | The cleavage site for oleoyl-CoA is within a short distance of a lipid acceptor. This revelation was made after a strong, lipid-like density in the central cavity in the cryo-EM data. Hydrophobic residues line this region and form a channel surrounding the lipid-like density. The channel itself has a bent, hydrophobic pathway that allows the binding of hydrophobic molecules. The bent architecture of this tunnel is likely how DGAT distinguishes acyl acceptors from other molecules, such as cholesterol. | ||
| - | === | + | ===Product Release=== |
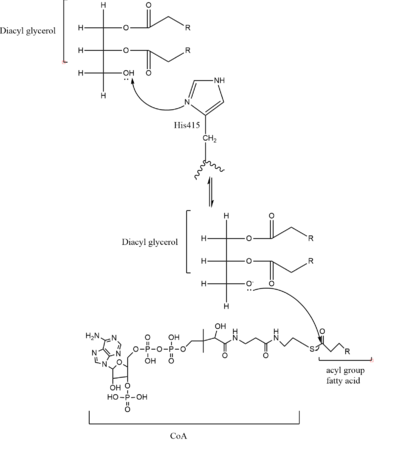
As previously mentioned, the Acyl-CoA molecule serves as the leaving group in the DGAT mechanism. This acyl-CoA molecule occupies the cytosolic tunnel, which has a bent architecture. The CoA moiety is at the cytosolic face, while the acyl chain extends through the center towards the endoplasmic reticulum lumen. The distal end of the acyl chain oleoyl-CoA interacts with DGAT deep within the hydrophobic channel, which suggests that the binding of longer acyl chains help accurately position the acyl-donor substrate for the reaction. As the acyl-CoA binds to DGAT, small conformational changes are seen in the active site region, specifically the His415 residue flips towards the endoplasmic reticulum-luminal side when acyl-CoA binds. This conformational change allows a new hydrogen bond to form and positions His415 near the thioester bond of the acyl-CoA. Therefore, the binding of acyl-CoA binding to DGAT results in small, but important, conformational changes in the active site that likely prime the enzyme for catalysis. | As previously mentioned, the Acyl-CoA molecule serves as the leaving group in the DGAT mechanism. This acyl-CoA molecule occupies the cytosolic tunnel, which has a bent architecture. The CoA moiety is at the cytosolic face, while the acyl chain extends through the center towards the endoplasmic reticulum lumen. The distal end of the acyl chain oleoyl-CoA interacts with DGAT deep within the hydrophobic channel, which suggests that the binding of longer acyl chains help accurately position the acyl-donor substrate for the reaction. As the acyl-CoA binds to DGAT, small conformational changes are seen in the active site region, specifically the His415 residue flips towards the endoplasmic reticulum-luminal side when acyl-CoA binds. This conformational change allows a new hydrogen bond to form and positions His415 near the thioester bond of the acyl-CoA. Therefore, the binding of acyl-CoA binding to DGAT results in small, but important, conformational changes in the active site that likely prime the enzyme for catalysis. | ||
Revision as of 20:03, 6 April 2021
Diacylglycerol Acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 https://www.proteinatlas.org/ENSG00000185000-DGAT1/pathology
- ↑ Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Justin Smith
- Eloi Bigirimana
- Leanne Price