Introduction
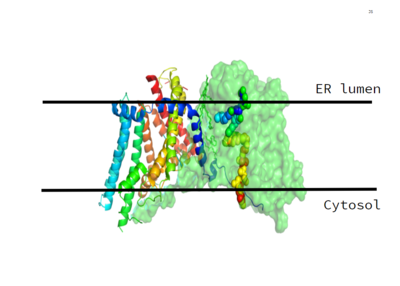
Figure 1. This image shows the location of the DGAT protein within the Endoplasmic Reticulum Membrane
DGAT, or Diacylglycerol Acyltransferase, is a polytopic endoplasmic reticulum membrane protein embedded within the membrane of the ER. DGAT is highly expressed in epithelial cells of the small intenstine of homo sapiens. It can also be found in the liver, where it helps synthesize fats for storage, and the female mammary glands, where it produces fat in the milk.
DGAT was originally discovered by its homology to Acyl-CoA cholesterol acyltransferases (ACAT) 1 and 2. The structure, catalytic mechanism of diacylglycerol acyltransferase, and how DGAT interacts with CoA was discovered using a Cryo-EM. The Cryo-EM map revealed that DGAT forms a dimer, with each subunit containing nine transmembrane helices. The N and C terminals of each helix are located on the cytosolic and luminal sides of the endoplasmic reticulum membrane respectively (Figure 1).
DGAT makes triglycerides from a diglyceride in plasma (Figure 2). In order to do this, DGAT uses two substrates: a fatty acyl-CoA and a diacylglycerol substrate. The basic mechanism consists of a lone pair on a hydroxyl group of glycerol attacking the carbon of the thioester bond of CoA. This results in the breakage of the thioester bond, and the attached acyl group attaches to the glycerol, creating a triglyceride.
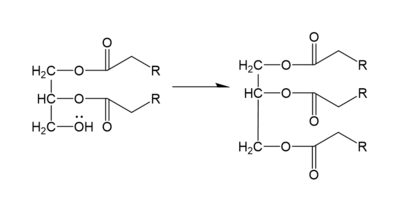
Figure 2. General schematic of DGAT's function.
Structure
DGAT consists of two domains, one cytoplasmic and one luminal. The cytoplasmic domain interacts with the interior of the cell and relays signals. The luminal domain senses misfolded proteins. The structure of DGAT consists of two protein chains, one ligand, two polymers, eighteen and zero beta sheets. The transmembrane helices form a large central cavity within the membrane that opens to the bilayer via a wide lateral gate. Through openings on the cytosolic and luminal sides of DGAT, this central cavity is also accessible. The majority of the transmembrane helices present within the structure also form a concave-shaped ridge on either side of the membrane. These aspects of the domain structure are deemed as the 'MBOAT core'. Within this core, a tunnel-like region, similar to a binding pocket, is also present. Access to the active site of DGAT by substrates is done through the lateral gate, which lies on the ER lumen side, within the membrane. This tunnel-like region is referred to as the .

Figure 3. Shows the location of the lateral gate as the entrance to the cytosolic tunnel via the ER lumen side of the membrane.
The DGAT dimer structure is formed primarily through many hydrogen-bonding interactions between the first 20 resolved residues (His69-Gly87). Hydrophobic interactions of the transmembrane helix region (Phe82-Ile98) with the other monomer also support the dimer structure formation. Additionally, there are four phospholipids present at the dimer interface that have been thought to contribute to the interactions between DGAT monomers.
Tunnels
Active Site
The active site of DGAT is located within the membrane, with the catalytic histidine residue (-represented in white) buried inside the central cavity. This central cavity serves as the catalytic site. The acyl-acceptor lipid substrates access the active site through the lateral gate within the membrane. The active site also contains and several nearby (including Asn378, Gln437, and Gln465) whose side chains are oriented towards the cavity center. These residues interact and create a hydrophilic channel within the active site. The His415 residue is also likely involved in catalysis, making it increasingly significant. In face, single mutations of His415 and Asn378 terminated DGAT activity. This suggests that the central cavity of DGAT within the membrane is the catalytic site.
Mechanism
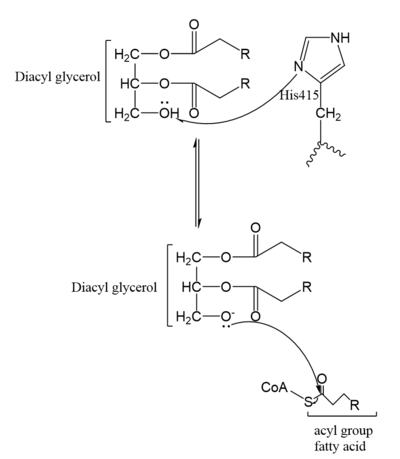
Figure 4. Mechanism for DGAT
In the DGAT mechanism, the diglyceride serves as the nucleophile. While the acyl group of the CoA enzyme serves as the electrophile. The lone pair on the last hydroxyl group present on the glycerol of the diglyceride attacks the thioester bond of the acyl-CoA enzyme. This attack breaks the sulfur-carbon bond, a weak bond that is easily breakable. This allows the acyl group of the acyl-CoA enzyme to attach to the diglyceride, creating a triglyceride. While the CoA group then serves as the leaving group.
The cleavage site for oleoyl-CoA is within a short distance of a lipid acceptor. This revelation was made after a strong, lipid-like density in the central cavity in the cryo-EM data. Hydrophobic residues line this region and form a channel surrounding the lipid-like density. The channel itself has a bent, hydrophobic pathway that allows the binding of hydrophobic molecules. The bent architecture of this tunnel is likely how DGAT distinguishes acyl acceptors from other molecules, such as cholesterol.
Product Release
As previously mentioned, the Acyl-CoA molecule serves as the leaving group in the DGAT mechanism. This acyl-CoA molecule occupies the cytosolic tunnel, which has a bent architecture. The CoA moiety is at the cytosolic face, while the acyl chain extends through the center towards the endoplasmic reticulum lumen. The distal end of the acyl chain oleoyl-CoA interacts with DGAT deep within the hydrophobic channel, which suggests that the binding of longer acyl chains help accurately position the acyl-donor substrate for the reaction. As the acyl-CoA binds to DGAT, small conformational changes are seen in the active site region, specifically the His415 residue flips towards the endoplasmic reticulum-luminal side when acyl-CoA binds. This conformational change allows a new hydrogen bond to form and positions His415 near the thioester bond of the acyl-CoA. Therefore, the binding of acyl-CoA binding to DGAT results in small, but important, conformational changes in the active site that likely prime the enzyme for catalysis.
Mutations
Mutations in the DGAT enzyme are particularly rare. One specific mutation discovered in the DGAT gene leads to congenital diarrhea, electrolyte derangements, protein-losing enteropathy and rickets. This homozygous recessive mutation prevents the expression of the gene and causes the DGAT enzyme to not be expressed. More specifically this mutation has been linked to a 3 base pair deletion at nucleotide position , resulting in an in-frame deletion of a phenylalanine residue at codon 338 maternally, and a C to G transversion at nucleotide position , resulting in a serine to arginine substitution at codon 420 paternally [1].




