We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madison Unger/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='6l47' size='340' frame='true' side='right' caption='Functioning dimer of ACAT' scene='87/877507/Dimer/5'> | <StructureSection load='6l47' size='340' frame='true' side='right' caption='Functioning dimer of ACAT' scene='87/877507/Dimer/5'> | ||
== Introduction == | == Introduction == | ||
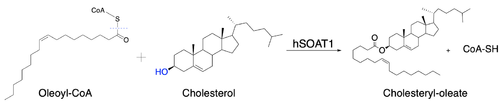
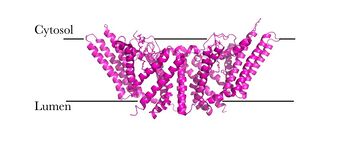
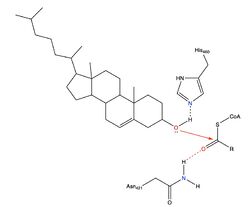
| - | [[Image:Newmechbigwords.png|500px|right|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase <scene name='87/877507/Dimer/5'>(ACAT)</scene>, also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: <scene name='87/877507/Dgat/2'>diacylglycerol acyltransferase</scene> ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) <ref>PMID:32433610</ref> and <scene name='87/877506/Goat/1'>ghrelin O-acyltransferase</scene>([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). [[Image:Cytosol lumen pic.jpg|360px|right|thumb|Figure 2: Orientation showing the cytosolic and lumen sides of the dimer]]<ref>PMID:19356147</ref> | + | [[Image:Newmechbigwords.png|500px|right|thumb|Figure 1: Function of ACAT]] Acyl-coenzyme A: cholesterol acyltransferase <scene name='87/877507/Dimer/5'>(ACAT)</scene>, also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain [http://en.wikipedia.org/wiki/Long-chain-fatty-acid%E2%80%94CoA_ligase fatty acyl CoA] and intracellular [http://en.wikipedia.org/wiki/Cholesterol cholesterol] to form the more hydrophobic cholesteryl ester for cholesterol storage (Fig.1). [http://en.wikipedia.org/wiki/Cholesteryl_ester Cholesteryl ester] is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein with a specific orientation (Fig.2), and ACAT is a part of the [http://en.wikipedia.org/wiki/MBOAT MBOAT] (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: <scene name='87/877507/Dgat/2'>diacylglycerol acyltransferase</scene> ([http://en.wikipedia.org/wiki/Diglyceride_acyltransferase DGAT]) <ref>PMID:32433610</ref> and <scene name='87/877506/Goat/1'>ghrelin O-acyltransferase</scene>([http://en.wikipedia.org/wiki/Ghrelin_O-acyltransferase GOAT]). [[Image:Cytosol lumen pic.jpg|360px|right|thumb|Figure 2: Orientation showing the cytosolic and lumen sides of the dimer]]<ref>PMID:19356147</ref> |
There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1<ref name="Qian">PMID:32433614</ref> and ACAT2<ref name="Cases">PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. | There have been two ACAT [http://en.wikipedia.org/wiki/Protein_isoform isoforms] discovered in mammals, ACAT1<ref name="Qian">PMID:32433614</ref> and ACAT2<ref name="Cases">PMID:9756919</ref>, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver. | ||
Revision as of 19:54, 19 April 2021
Human Acyl-Coenzyme A
| |||||||||||
References
- ↑ Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Moorthy PS, Neelagandan K, Balasubramanian M, Ponnuswamy MN. Purification, Crystallization and Preliminary X-Ray Diffraction Studies on Goat (Capra hircus) Hemoglobin - A Low Oxygen Affinity Species. Protein Pept Lett. 2009;16(4):454-6. PMID:19356147
- ↑ 3.0 3.1 3.2 3.3 3.4 Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ 4.0 4.1 Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV Jr. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998 Oct 9;273(41):26755-64. doi: 10.1074/jbc.273.41.26755. PMID:9756919 doi:http://dx.doi.org/10.1074/jbc.273.41.26755
- ↑ 5.0 5.1 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Liu J, Chang CC, Westover EJ, Covey DF, Chang TY. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem J. 2005 Oct 15;391(Pt 2):389-97. doi: 10.1042/BJ20050428. PMID:15992359 doi:http://dx.doi.org/10.1042/BJ20050428
- ↑ Rogers MA, Liu J, Song BL, Li BL, Chang CC, Chang TY. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J Steroid Biochem Mol Biol. 2015 Jul;151:102-7. doi: 10.1016/j.jsbmb.2014.09.008., Epub 2014 Sep 12. PMID:25218443 doi:http://dx.doi.org/10.1016/j.jsbmb.2014.09.008
- ↑ Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer's disease: the lipid connection. J Neurochem. 2007 Nov;103 Suppl 1:159-70. doi: 10.1111/j.1471-4159.2007.04715.x. PMID:17986151 doi:http://dx.doi.org/10.1111/j.1471-4159.2007.04715.x
- ↑ Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S, Konieczny SF, Ratliff TL, Liu X, Xie J, Cheng JX. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016 Dec 15;35(50):6378-6388. doi: 10.1038/onc.2016.168. Epub 2016 May , 2. PMID:27132508 doi:http://dx.doi.org/10.1038/onc.2016.168
- ↑ Rudel LL, Shelness GS. Cholesterol esters and atherosclerosis-a game of ACAT and mouse. Nat Med. 2000 Dec;6(12):1313-4. doi: 10.1038/82110. PMID:11100106 doi:http://dx.doi.org/10.1038/82110
Student Contributors
- Leah Goehring
- Gabby Smith
- Anna Campbell