User:Brianna Avery/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
====Binding Pocket==== | ====Binding Pocket==== | ||
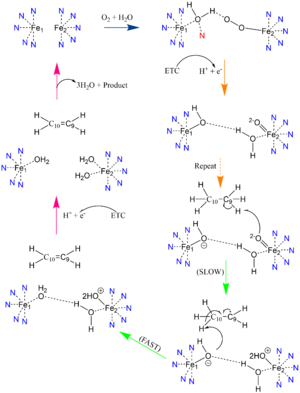
| - | SCD-1 consists of a hydrophobic <scene name='87/877504/Baseline_structure/3'>V-shaped tunnel</scene> deep within the protein that the substrates will enter <ref name="Larochelle">DOI: 10.1038/nsmb.3049</ref>. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals. | + | SCD-1 consists of a hydrophobic <scene name='87/877504/Baseline_structure/3'>V-shaped tunnel</scene> deep within the protein that the substrates will enter <ref name="Larochelle">DOI: 10.1038/nsmb.3049</ref>. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals. Precise placement of the <scene name='87/877504/Zn_h_bond_stabilization_2/7'>C9-C10</scene> atoms near the two iron metals provides the tunnel with regioselectivity and stereospecificity, stabilizing the substrate for oxygen to extract the hydrogens in order to form the double bond. The kink is formed by two conserved <scene name='87/877504/Trp_thr_creation_of_kink/1'>Trp149 and Thr257 residues</scene> <ref name="Bai" />. It is at this kink of the tunnel where desaturation occurs. |
====Metal Cations==== | ====Metal Cations==== | ||
| Line 29: | Line 29: | ||
==Function== | ==Function== | ||
| - | Palmitoyl and Stearoyl CoA are substrates of SCD1. These substrates enter the hydrophobic V-shaped tunnel inside SCD1. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals | + | Palmitoyl and Stearoyl CoA are substrates of SCD1. These substrates enter the hydrophobic V-shaped tunnel inside SCD1. The tunnel is regioselective and stereospecific such that the substrate’s binding site lines up C9 and C10 at the kink of the V-shaped tunnel with the di-iron center that consists of an oxygen molecule bound to one of the metals. It is at this kink of the tunnel that desaturation occurs. Hydrogens are removed at the C9, then C10 to introduce the double bond through mechanism (link to mechanism section?). |
====Desaturation Mechanism==== | ====Desaturation Mechanism==== | ||
Revision as of 20:13, 19 April 2021
Desaturation of Fatty Stearoyl-CoA by SCD
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers (Basel). 2019 Jul 5;11(7). pii: cancers11070948. doi:, 10.3390/cancers11070948. PMID:31284458 doi:http://dx.doi.org/10.3390/cancers11070948
- ↑ 3.0 3.1 Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
- ↑ Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, Li K, Sang BC. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat Struct Mol Biol. 2015 Jul;22(7):581-5. doi: 10.1038/nsmb.3049. Epub 2015 Jun , 22. PMID:26098317 doi:http://dx.doi.org/10.1038/nsmb.3049
- ↑ Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do KA, Fraser Symmans W, Pusztai L, Hortobagyi GN, Mills GB, Meric-Bernstam F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat. 2013 Jan;137(1):319-27. doi: 10.1007/s10549-012-2354-4. , Epub 2012 Dec 4. PMID:23208590 doi:http://dx.doi.org/10.1007/s10549-012-2354-4
- ↑ Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017 Mar 2;20(3):303-314.e5. doi: 10.1016/j.stem.2016.11.004., Epub 2016 Dec 29. PMID:28041894 doi:http://dx.doi.org/10.1016/j.stem.2016.11.004
Student Contributors
- Brianna M. Avery
- William J. Harris III
- Emily M. Royston