We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Maggie Stopa/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | |||
=Lipoprotein Lipase LPL= | =Lipoprotein Lipase LPL= | ||
<StructureSection load='6ob0' size='350' side='right' caption='Lipoprotein Lipase PDB' scene='87/877513/Original_scene/1'> | <StructureSection load='6ob0' size='350' side='right' caption='Lipoprotein Lipase PDB' scene='87/877513/Original_scene/1'> | ||
| Line 6: | Line 7: | ||
==Structural Overview== | ==Structural Overview== | ||
| - | <scene name='87/877513/Original_scene/1'>LPL</scene> is assumed to only be active as a <scene name='87/877513/Lpl_dimer/ | + | <scene name='87/877513/Original_scene/1'>LPL</scene> is assumed to only be active as a <scene name='87/877513/Lpl_dimer/2'>homodimer</scene>, however, previous studies have argued that the lipase can be active in its monomeric form. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6442593/) The N-terminal domain of lipoprotein lipase is known to consist of an alpha/beta hydrolase domain, which is composed of six alpha helices and ten beta-strands. This domain creates an <scene name='87/877513/Alpha-beta_hydrolase_domain/1'>alpha/beta hydrolase fold</scene>. The C-terminal domain of lipoprotein lipase is composed of twelve beta strands which form a “<scene name='87/877513/Barrel_domain/3'>barrel domain</scene>”. |
==Mechanism== | ==Mechanism== | ||
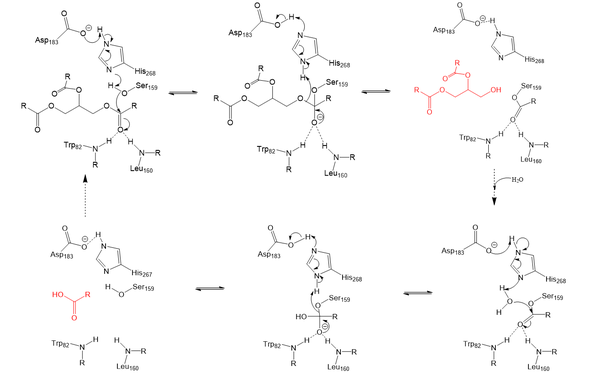
Lipoprotein Lipase functions to catalyze the hydrolysis of one [https://en.wikipedia.org/wiki/Ester ester bond] of triglycerides. It does this by utilizing a simple [https://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] mechanism, in which it uses a <scene name='87/877513/Catalytic_triad/3'>catalytic triad</scene> composed of Asp183, His268, and Ser159 to catalyze the hydrolysis. His268 serves as a base catalyst by deprotonation of Ser159, which can then serve as the [https://en.wikipedia.org/wiki/Nucleophile nucleophile]. The transition state of the catalysis is stabilized by the <scene name='87/877516/Oxyanion_hole_master/1'>oxyanion hole</scene> composed of Trp82 and Leu160. The hydrolysis results in the formation of one free fatty acid and a glycerol with two fatty acid tails. | Lipoprotein Lipase functions to catalyze the hydrolysis of one [https://en.wikipedia.org/wiki/Ester ester bond] of triglycerides. It does this by utilizing a simple [https://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] mechanism, in which it uses a <scene name='87/877513/Catalytic_triad/3'>catalytic triad</scene> composed of Asp183, His268, and Ser159 to catalyze the hydrolysis. His268 serves as a base catalyst by deprotonation of Ser159, which can then serve as the [https://en.wikipedia.org/wiki/Nucleophile nucleophile]. The transition state of the catalysis is stabilized by the <scene name='87/877516/Oxyanion_hole_master/1'>oxyanion hole</scene> composed of Trp82 and Leu160. The hydrolysis results in the formation of one free fatty acid and a glycerol with two fatty acid tails. | ||
| - | [[Image:LPL_final_Mechanism.png| | + | [[Image:LPL_final_Mechanism.png|600 px|center|thumb|Serine hydrolase mechanism utilized by LPL to catalyze the breakdown of one ester bond of a triglyceride. Compounds colored red are the products of the hydrolysis.]] |
| - | + | ||
== Relevance & Disease == | == Relevance & Disease == | ||
| Line 24: | Line 24: | ||
Original look: <scene name='87/877513/Original_scene/1'>LPL</scene> | Original look: <scene name='87/877513/Original_scene/1'>LPL</scene> | ||
| - | GPI/LPL interface: <scene name='87/877513/Hydrophobic_interface/1'>hydrophobic interface</scene> | + | GPI/LPL interface (w/labels): <scene name='87/877513/Hydrophobic_interface-labeled/1'>hydrophobic interface</scene> |
| - | Calcium ion stabilization:<scene name='87/877513/ | + | |
| + | |||
| + | |||
| + | |||
| + | Calcium ion stabilization:<scene name='87/877513/Calcium_stabilization_-labeled/1'>calcium ion stabilization</scene> | ||
<scene name='87/877514/Lipid_binding_and_lid/1'>lipid binding region</scene> | <scene name='87/877514/Lipid_binding_and_lid/1'>lipid binding region</scene> | ||
| - | <scene name='87/877513/Hydrophobic_interface-labeled/1'>hydrophobic interface</scene> | ||
| - | Lid: <scene name='87/877514/Lid_region_final/1'>Lid Region</scene> | + | |
| - | < | + | Lid: <scene name='87/877514/Lid_region_final/1'>Lid Region</scene> |
| - | < | + | |
| + | |||
| + | == References == | ||
| + | <references/> | ||
| + | <ref name=”Arora”>PMID:31072929</ref> | ||
| + | <ref name=”Birrane”>PMID:30559189</ref> | ||
| + | <ref name=”Davies”>PMID:20620994</ref> | ||
| + | <ref name=”Beigneux”>PMID:30850549</ref> | ||
| + | <ref name=”Mead”>PMID:12483461</ref> (LPL GENERAL REFERENCE) | ||
| + | <ref name=”Eckel”>PMID:2648155</ref> (LPL GENERAL REFERENCE BOOK IF NEEDED) | ||
| + | |||
| + | |||
| + | ==Student Contributors== | ||
| + | |||
| + | Giselle Flores | ||
| + | |||
| + | Dustin Soe | ||
| + | |||
| + | Maggie Stopa | ||
Revision as of 21:42, 23 April 2021
Lipoprotein Lipase LPL
| |||||||||||