We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jacob Holt/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Structural Overview == | == Structural Overview == | ||
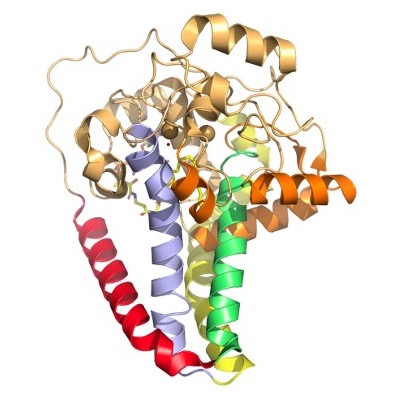
| - | SCD1 is a [https://en.wikipedia.org/wiki/Transmembrane protein transmembrane protein] (4 helices in membrane, 8 helices in cytoplasm) that acquires electrons via an electron transport chain which includes [https://en.wikipedia.org/wiki/Cytochrome_b5_reductase cytochrome b5 reductase] and [https://en.wikipedia.org/wiki/Cytochrome_b5 cytochrome b5] . The electrons are transferred via a ternary complex and accepted by SCD1 by the iron metal ions<ref name="Shen" />. SCD1 has 8 helices that are hydrophobic, 4 helices that are hydrophilic, and 3 helices that are amphipathic<ref name="Bai" /><ref name="Shen" />. There are two Fe+2 metal ions within the structure of SCD1 that were determined by x-ray fluorescence chromatography [https://en.wikipedia.org/wiki/X-ray_fluorescence x-ray fluorescense]<ref name="Shen" />. [[Image:colorful2.jpg|400 px|thumb|Figure | + | SCD1 is a [https://en.wikipedia.org/wiki/Transmembrane protein transmembrane protein] (4 helices in membrane, 8 helices in cytoplasm) that acquires electrons via an electron transport chain which includes [https://en.wikipedia.org/wiki/Cytochrome_b5_reductase cytochrome b5 reductase] and [https://en.wikipedia.org/wiki/Cytochrome_b5 cytochrome b5] . The electrons are transferred via a ternary complex and accepted by SCD1 by the iron metal ions<ref name="Shen" />. SCD1 has 8 helices that are hydrophobic, 4 helices that are hydrophilic, and 3 helices that are amphipathic<ref name="Bai" /><ref name="Shen" />. There are two Fe+2 metal ions within the structure of SCD1 that were determined by x-ray fluorescence chromatography [https://en.wikipedia.org/wiki/X-ray_fluorescence x-ray fluorescense]<ref name="Shen" />. |
| + | [[Image:colorful2.jpg|400 px|thumb|Figure 2. Hydrophobicity of each of the 12 helices found in SCD. red, blue, yellow, and green represent helices found in the transmembrane region. Orange helices represent helices found on the surface of the membrane. Pale yellow helices represent the hydrophilic helices.]] | ||
| + | |||
| + | [[Image:colored_helices.jpg|420 px|thumb|Figure 3. Colored helices based on hydrophobicity. Red, green, yellow, and blue represent the transmembrane helices. Orange represents the helices found on the surface of the membrane, and tan represents the helices found in the cytoplasm.]] | ||
| Line 75: | Line 78: | ||
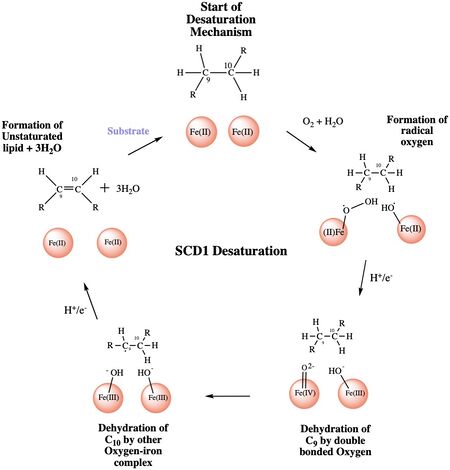
[[Image:SCD1_New.jpeg|450 px|right|thumb|Figure 4: A proposed mechanism for SCD1 desaturase. The catalytic water molecule reacts with Fe2+ and O2 to create oxygen radicals on the iron ions. Electrons are brought in via an electron transport chain; this lowers the oxidation state of the iron ions and forms the reactive species. The first hydrogen is abstracted creating a radical intermediate that is then deprotonated again to make the final desaturated product. Electrons are transferred in again to allow for the iron ions to go back to their original oxidation state because enzymes must end a reaction in the same state, they started it. Figure was a modification of scheme 1 proposed by Yu M. and Chen S.5 ]] | [[Image:SCD1_New.jpeg|450 px|right|thumb|Figure 4: A proposed mechanism for SCD1 desaturase. The catalytic water molecule reacts with Fe2+ and O2 to create oxygen radicals on the iron ions. Electrons are brought in via an electron transport chain; this lowers the oxidation state of the iron ions and forms the reactive species. The first hydrogen is abstracted creating a radical intermediate that is then deprotonated again to make the final desaturated product. Electrons are transferred in again to allow for the iron ions to go back to their original oxidation state because enzymes must end a reaction in the same state, they started it. Figure was a modification of scheme 1 proposed by Yu M. and Chen S.5 ]] | ||
| + | |||
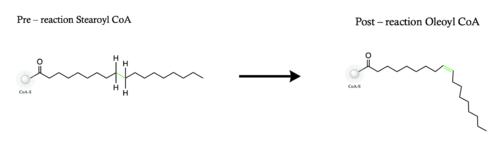
The mechanism used by the SCD1 enzyme is different than most desaturase enzymes because it does not use an oxo-bridge<ref name="Shen" />. This mechanism begins with the addition of O2 and H2O which react with the Fe2+ ions to create oxygen radicals on the iron ions<ref name="Shen" />. Then an electron/proton pair is brought in via the electron transport chain; this increases the oxidation of both iron ions, gets rid of the radicals, and creates an active iron-oxyl molecule<ref name="Shen" />. The iron-oxyl molecule is reactive enough, due to the change in the oxidation state, to pull off the first hydrogen on carbon 9<ref name="Yu" />. An unstable radical intermediate of the 18-carbon acyl-CoA ligand is formed which reacts with the other iron-O molecule, in the +3 state, to pull of the second hydrogen and form the final product (figure)<ref name="Yu" />. Another electron/proton pair is brought in to create three H2O molecules and to take the iron ions back down to their original oxidation state of +2<ref name="Yu" />. | The mechanism used by the SCD1 enzyme is different than most desaturase enzymes because it does not use an oxo-bridge<ref name="Shen" />. This mechanism begins with the addition of O2 and H2O which react with the Fe2+ ions to create oxygen radicals on the iron ions<ref name="Shen" />. Then an electron/proton pair is brought in via the electron transport chain; this increases the oxidation of both iron ions, gets rid of the radicals, and creates an active iron-oxyl molecule<ref name="Shen" />. The iron-oxyl molecule is reactive enough, due to the change in the oxidation state, to pull off the first hydrogen on carbon 9<ref name="Yu" />. An unstable radical intermediate of the 18-carbon acyl-CoA ligand is formed which reacts with the other iron-O molecule, in the +3 state, to pull of the second hydrogen and form the final product (figure)<ref name="Yu" />. Another electron/proton pair is brought in to create three H2O molecules and to take the iron ions back down to their original oxidation state of +2<ref name="Yu" />. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 93: | Line 115: | ||
== Biological Relevance == | == Biological Relevance == | ||
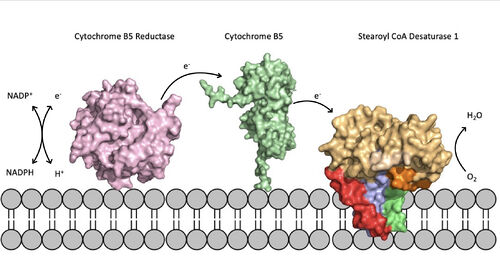
| - | [[Image:scd_in_membrane.jpeg|500 px|thumb|Figure | + | [[Image:scd_in_membrane.jpeg|500 px|thumb|Figure 5. Position of SCD within the biological membrane. It is part of an electron transport chain involving cytochrome b5 reductase and cytochrome b5 to allow for the activation of the catalytic molecule coordinated by the two ions in the center of SCD]]Absence or a deficit of SCD1 in the body is associated with obesity and insulin resistant which is a main cause of Type II diabetes [https://en.wikipedia.org/wiki/Type_2_diabetes type 2 diabetes]<ref name="Shen" />. Cancer sites in the body tend to show a much higher expression rate of SCD1<ref name="Shen" />. Focusing on SCD1 as a drug target could lead to advancements in treatment of obesity, diabetes, and other metabolic diseases<ref name="Bai" />. The ligand structure was determined by using Zn2+ metal ions and product structure was determined using Fe2+ ions<ref name="Shen" />. |
Revision as of 21:28, 23 April 2021
Desaturation of Fatty Acids using Stearoyl-CoA Desaturase-1 Enzyme
| |||||||||||
Student Contributions
Carson Maris, Jess Kersey, Jacob Holt