We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
=== Active Site === | === Active Site === | ||
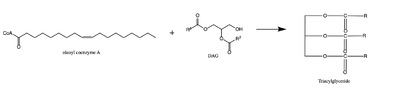
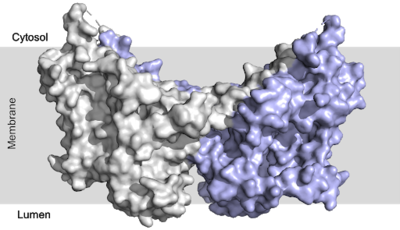
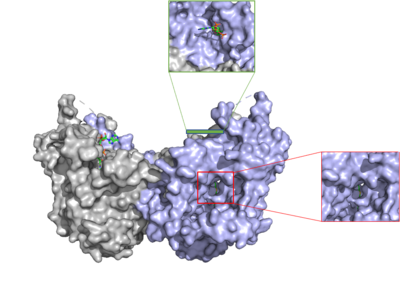
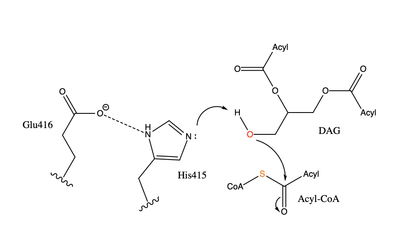
The active site of DGAT is in the transmembrane region of the enzyme. When it is in its <scene name='87/877557/His_no_oleoyl/3'>unbound</scene> state and no oleoyl-CoA is in its tunnel, Met434 hydrogen bonds to the catalytic histidine, His415, which stabilizes the conformation. There are no major conformational changes that take place upon oleoyl-CoA binding into the cytosolic tunnel, however, several key residues change conformation to allow for correct positioning | The active site of DGAT is in the transmembrane region of the enzyme. When it is in its <scene name='87/877557/His_no_oleoyl/3'>unbound</scene> state and no oleoyl-CoA is in its tunnel, Met434 hydrogen bonds to the catalytic histidine, His415, which stabilizes the conformation. There are no major conformational changes that take place upon oleoyl-CoA binding into the cytosolic tunnel, however, several key residues change conformation to allow for correct positioning | ||
| - | of the ligand. In its <scene name='87/ | + | of the ligand. In its <scene name='87/878228/Active_site/1'>bound</scene>conformation, a hydrogen bond is formed between His415 and Gln465 which stabilizes the histidine and allows it to be positioned near the thioester bond of the oleoyl-CoA.<ref name="Sui">PMID:32433611</ref> His415 is now positioned to be able to interact with the DAG that enters through the lateral tunnel perpendicular to the cytosolic tunnel oleoyl-CoA enters through. <scene name='87/877557/Asn378/1'>Asn378</scene> has been hypothesized to be important in holding the DAG in a proper orientation to be able to interact with the oleoyl-CoA and undergo synthesis into a triglyceride. <ref name="Sui">PMID:32433611</ref> |
=== Mechanism === | === Mechanism === | ||
Revision as of 15:27, 24 April 2021
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
- ↑ 6.0 6.1 Gluchowski, N. L., Chitraju, C., Picoraro, J. A., Mejhert, N., Pinto, S., Xin, W., Kamin, D. S., Winter, H. S., Chung, W. K., Walther, T. C., & Farese, R. V., Jr (2017). Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. Journal of lipid research, 58(6), 1230–1237. https://doi.org/10.1194/jlr.P075119
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko