Introduction
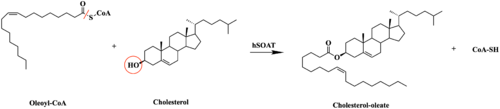
Figure 1. Esterification Reaction of Oleoyl-CoA and Cholesterol catalyzed by SOAT.
Sterol O-acyltransferase(SOAT), otherwise known as Acyl-coenzyme A:cholesterol acyltransferase(ACAT), is the first discovered member of the membrane-bound O-acyl
transferase or MBOAT enzyme group. MBOAT enzymes are responsible for the transfer of
acyl chains onto multiple types of substrates within the cell. There are 11 MBOAT enzyme types that can be found in humans, all of which serve a different function in the overall makeup of human biology.
[1]
SOAT specifically catalyzes the esterification of cholesterol for efficient storage within the cell. Cholesterol is a type of membrane lipid that is responsible for controlling the fluidity and integrity of the membrane, along with other important biological processes. When there are high concentrations of cholesterol in the cell, cholesteryl esters can be formed for storage within the membrane.[1]
Structure
Tertiary Structure
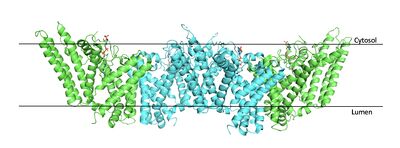
Figure 2. Tetramer unit of SOAT shown as it sits within the membrane. Each dimer is composed of a green and blue chain with the corresponding monomer chains colored the same.
PBD 6P2P The overall structure of the enzyme is a structure or a dimer of dimers. The functional building block of SOAT is a which is made up of two identical units. The residues that form the dimer interface are mostly hydrophobic and interact with each other in a shape-complementary manner. Mutating residues within the dimer interface reduced the dimers to monomer fractions, indicating that the dimeric architecture is important for the activity of the enzyme. Each monomer is organized into 9 .
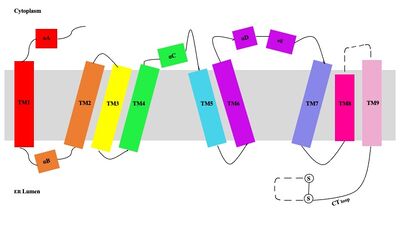
Figure 3. Labeled helices of SOAT within the membrane
The dimerization of SOAT is mainly mediated by extensive
van der Waals interactions between TM1 in one protomer and the
lumenal segment of TM6 and the
cytosolic segment of TM9 in the other. TM1, TM5, TM6 and TM9 from the two protomers enclose a deep hydrophobic pocket that is open to the lumenal side. Numerous hydrophobic residues on TM6 and TM9 from one protomer contact those on TM1 from the other protomer. On the intracellular side, hydrophobic residues on IH1 of each protomer interact with each other to stabilize the dimer.
[2]
Tunnel System
A main structural element of this enzyme is the tunnel systems.

Figure 4. 2D layout of the SOAT tunnel system. The orientation of the tunnels shows the C tunnel opening to the cytosol and the L tunnel opening to the lumen. The T tunnel opens into the membrane, but is not quite the 90 degree shown in the 2D image.
There are 3 main tunnels in each monomer: the cytosolic (C) tunnel opening to the cytosol, the transmembrane(T) tunnel opening to the membrane, and the lumenal (L) tunnel opens to the lumen.
[2] The C tunnel opens to the cytosol of the cell and is the entrance site for the Acyl CoA into the active site. Surface representations of SOAT indicate that there are 2 alpha helices that block the entrance to the C tunnel, therefore a conformational change needs to occur to move the 2 helices so the substrate can enter the tunnel. The T tunnel opens into the membrane and is where cholesterol enters to have access to the active site. The two substrates are catalyzed by the H460 in the active site to form the cholesteryl ester. The products then leave via different pathways. The CoA-SH in the C tunnel leaves via that tunnel and is released back into the cytosol. The cholesteryl ester then leaves via either the T tunnel into the membrane or through the L tunnel into the lumen of the cell.
[2]
Active Site
The substrate, , is shown bound to SOAT to visualize the binding pocket and the 3 main residues that are essential for the catalytic activity. work to stabilize the substrates as well as serve other roles in the mechanism of action. Histidine is commonly used as the catalytic base for many acyl transferase reactions. H460 is highly conserved across a variety of species and is essential for SOAT catalysis. It is assumed to be the most important catalytic residue.[1] Mutating this histidine at position 460 to alanine completely abolishes enzymatic activity, indicating its essential role in the catalytic mechanism.[2] SOAT activity also relies on several other highly conserved residues within the interior of the central cavitity. This high preservation of residues suggests that the local environment plays a major role in SOAT activity.
Catalytic Mechanism
The distal-most nitrogen on H460 acts as a base catalyst to deprotonate the hydroxyl group of a cholesterol molecule. This leaves the cholesterol oxygen with a negative charge, making it a good nucleophile. The
nucleophilic oxygen attacks the Acyl CoA substrate at the carbonyl carbon, kicking electron density up to the carbonyl oxygen. Shown in brackets, the transition state is stabilized by N421 and newly protonated H460.
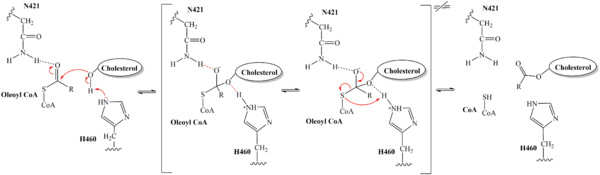
Figure 5. Mechanism for the esterification reaction of SOAT with arrow pushing.
From the transition state, excess electron density on the carbonyl oxygen is collapsed back into a double bond. This causes the bond between the carbonyl carbon and sulfur to break, shifting electron density to the sulfur atom. To complete the mechanism, the negatively charged sulfur would reclaim the hydrogen from protonated H460. Acyl CoA would exit the active site as a leaving group, leaving its R group attached to cholesterol in the form of a cholesterol ester.
It should be noted that this mechanism is largely hypothesized. Further analysis is needed to confirm the proposed steps. Additionally, mutations of W420A rendered the SOAT enzyme nonfunctional, indicating that it must be essential for catalytic activity. However, its role in the mechanism was not explicitly hypothesized. We believe that it plays a role in substrate binding through with CoenzymeA.
Inhibitors
SOAT activity is inhibited by CI-976, depending on the concentration and exposure of the inhibitor to the SOAT enzyme. When exposed, CI-976 locks itself in the of the enzyme. The trimethoxyphenyl head can be found interacting with the catalytic residues . The interactions with these residues as well as the location of the trimethoxyphenol head indicate that CI-976 inhibits the SOAT enzyme in a competitive manner by preventing the substrate, oleyl-CoA, from entering the catalytic center via the C tunnel. Similar to the interactions with the substrates, mutating those key catalytic residues, N421A, H460A, and H460N, result in a smaller effect of the inhibitor on the thermostability of the enzyme. [1]
Biological Relevance
SOAT can actually use multiple sterols as substrates and activators. Because of its functional importance, SOAT is a potential drug target for Alzheimer’s disease, atherosclerosis, and several types of cancers.
Alzheimer’s disease (AD) is a progressive disease that severely hinders a person’s memory and other cognitive functions. AD is the result of a significant increase in beta-amyloid (Aβ) peptide concentration. [3] Previous studies have found that the amount and distribution of intracellular cholesterol plays an important role in regulating Aβ production.[4] Therefore, SOAT inhibition could be an effective therapy for treating AD because it would reduce cholesteryl ester formation in the brain and help lower Aβ generation as well. [3] [4]
Another disease SOAT inhibition could help treat is atherosclerosis. Buildup of cholesteryl esters from SOAT catalysis has been shown to be partially responsible for foam cell formation, one of the major indicators of atherosclerosis. Consequently, SOAT inhibitors have been studied as potential drug targets for this disease.[5]
Increased expression of SOAT and abnormal accumulation of cholesteryl esters has also been found in multiple cancers including ovarian cancer. Therefore, inhibiting SOAT and exhausting cholesteryl ester concentrations has shown to have anti-tumor effects in terms of monitoring apoptosis, cell proliferation, and migration and invasion properties. Therapies that target SOAT regulation and expression levels could thus lead to potential treatments for ovarian and other types of cancer.[6]
In general, targeting SOAT could be an effective means for treating various diseases. Aberrant quantities of cholesteryl esters seem to hinder various cellular processes; thus, inhibiting SOAT expression and functionality could help reduce these adverse effects. Overall, SOAT plays an important role in cholesterol homeostasis and future research of this enzyme could lead to the discovery of therapeutic treatments for different illnesses.





