User:Haylie Moehlenkamp/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 46: | Line 46: | ||
===Alzheimer's Disease=== | ===Alzheimer's Disease=== | ||
| - | [https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Alzheimer's Disease]is a neurodegenerative disease characterized by accumulation of extracellular plaques that cause interferences with memory retrieval. These plaques are made up of [https://en.wikipedia.org/wiki/Amyloid_beta] (Aβ) peptides which are products of the cleavage of [https://en.wikipedia.org/wiki/Amyloid-beta_precursor_protein#:~:text=Amyloid%2Dbeta%20precursor%20protein%20(APP,antimicrobial%20activity%2C%20and%20iron%20export.] (hAPP) <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. Within the cells, there is an accumulation of hyperphosphorylated [https://en.wikipedia.org/wiki/Tau_protein] <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. Research has shown that the concentration of cholesterol within cells can affect the production of Aβ <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. As the concentration of cholesterol in the endoplasmic reticulum of neurons increases, hAPP is downregulated <ref name "Chang">doi:10.1002/iub.305</ref><ref name="Shibuya"> PMID:26669800</ref>. Inhibition of ACAT1 would lead to higher concentrations of cholesterol in the cells, signaling downregulation of hAPP. Less hAPP available decreases the amount of Aβ peptides being produced which then reduces the available Aβ peptides that form the extracellular plaques associated with Alzheimer's Disease <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref> | + | [https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Alzheimer's Disease]is a neurodegenerative disease characterized by accumulation of extracellular plaques that cause interferences with memory retrieval. These plaques are made up of [https://en.wikipedia.org/wiki/Amyloid_beta Amyloid Beta] (Aβ) peptides which are products of the cleavage of [https://en.wikipedia.org/wiki/Amyloid-beta_precursor_protein#:~:text=Amyloid%2Dbeta%20precursor%20protein%20(APP,antimicrobial%20activity%2C%20and%20iron%20export. Human Amyloid-Beta Precursor Protein] (hAPP) <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. Within the cells, there is an accumulation of hyperphosphorylated [https://en.wikipedia.org/wiki/Tau_protein Tau] protein <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. Research has shown that the concentration of cholesterol within cells can affect the production of Aβ <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. As the concentration of cholesterol in the endoplasmic reticulum of neurons increases, hAPP is downregulated <ref name "Chang">doi:10.1002/iub.305</ref><ref name="Shibuya"> PMID:26669800</ref>. Inhibition of ACAT1 would lead to higher concentrations of cholesterol in the cells, signaling downregulation of hAPP. Less hAPP available decreases the amount of Aβ peptides being produced which then reduces the available Aβ peptides that form the extracellular plaques associated with Alzheimer's Disease <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. |
Revision as of 21:57, 25 April 2021
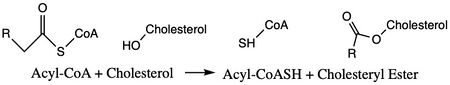
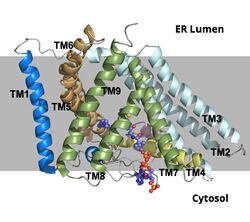
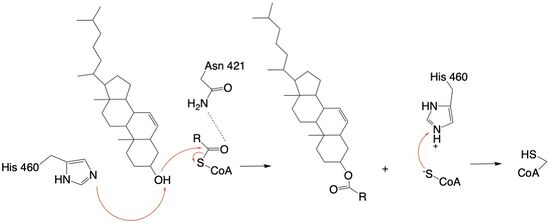
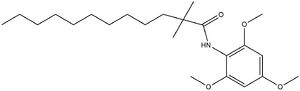
Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1)
| |||||||||||
References
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 4.0 4.1 4.2 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
- ↑ Vaziri ND, Liang KH. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004 Jul 27;110(4):419-25. doi: 10.1161/01.CIR.0000136023.70841.0F. , Epub 2004 Jul 19. PMID:15262831 doi:http://dx.doi.org/10.1161/01.CIR.0000136023.70841.0F
- ↑ Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1262-7. doi: 10.1073/pnas.0336398100., Epub 2003 Jan 21. PMID:12538880 doi:http://dx.doi.org/10.1073/pnas.0336398100
- ↑ 8.0 8.1 8.2 8.3 8.4 Chang TY, Chang CC, Bryleva E, Rogers MA, Murphy SR. Neuronal cholesterol esterification by ACAT1 in Alzheimer's disease. IUBMB Life. 2010 Apr;62(4):261-7. doi: 10.1002/iub.305. PMID:20101629 doi:http://dx.doi.org/10.1002/iub.305
- ↑ 9.0 9.1 9.2 9.3 9.4 Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. PMID:26669800 doi:http://dx.doi.org/10.4155/fmc.15.161
Student Contributors
- Haylie Moehlenkamp, Megan Fleshman, Tori Templin