User:Brianna Avery/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
[https://en.wikipedia.org/wiki/Stearoyl-CoA_desaturase-1 Stearoyl-CoA Desaturase-1] (SCD1) is an integral membrane protein located in the endoplasmic reticulum and is conserved across all eukaryotes <ref name="Bai">DOI: 10.1038/nature14549</ref>. The SCD-1 pictured is expressed in Mus musculus (house mouse). The human homolog, SCD1, shares 85% sequence identity with all four SCD’s found in M. musculus (SCD1-SCD4). The expression of SCD1 is seen mainly in the liver and brain <ref name="Dobrzyn">PMID: 31284458</ref>. | [https://en.wikipedia.org/wiki/Stearoyl-CoA_desaturase-1 Stearoyl-CoA Desaturase-1] (SCD1) is an integral membrane protein located in the endoplasmic reticulum and is conserved across all eukaryotes <ref name="Bai">DOI: 10.1038/nature14549</ref>. The SCD-1 pictured is expressed in Mus musculus (house mouse). The human homolog, SCD1, shares 85% sequence identity with all four SCD’s found in M. musculus (SCD1-SCD4). The expression of SCD1 is seen mainly in the liver and brain <ref name="Dobrzyn">PMID: 31284458</ref>. | ||
| - | SCD is an enzyme which catalyzes desaturation of a double bond within a fatty acid hydrocarbon chain. The addition of a double bond is necessary for the biosynthesis of monounsaturated fatty acids such as: cholesterol, phospholipids, and triglycerides. The enzyme’s main function is [https://en.wikipedia.org/wiki/Lipid_metabolism#:~:text=%2B-,Lipid%20biosynthesis,the%20organisms%20through%20various%20pathways. lipid biosynthesis] as well as regulating gene expression for [https://en.wikipedia.org/wiki/Lipogenesis#:~:text=Lipogenesis%20is%20the%20metabolic%20process,packaged%20within%20cytoplasmic%20lipid%20droplets. lipogensis] <ref name="Bai" />.[[Image:Screen_Shot_2021-04-24_at_9.00.21_PM.png|275 px|left|thumb|Saturated vs. Unsaturated Fatty Acid Chain]] | + | SCD is an enzyme which catalyzes desaturation of a double bond within a fatty acid hydrocarbon chain. The addition of a double bond is necessary for the biosynthesis of monounsaturated fatty acids such as: cholesterol, phospholipids, and triglycerides. The enzyme’s main function is [https://en.wikipedia.org/wiki/Lipid_metabolism#:~:text=%2B-,Lipid%20biosynthesis,the%20organisms%20through%20various%20pathways. lipid biosynthesis] as well as regulating gene expression for [https://en.wikipedia.org/wiki/Lipogenesis#:~:text=Lipogenesis%20is%20the%20metabolic%20process,packaged%20within%20cytoplasmic%20lipid%20droplets. lipogensis] <ref name="Bai" />. SCD1 is regulated by transcription and its promoter has multiple binding sites for transcription factors that assist with [[Image:Screen_Shot_2021-04-24_at_9.00.21_PM.png|275 px|left|thumb|Saturated vs. Unsaturated Fatty Acid Chain]]regulation of lipogenesis <ref name="Dobrzyn" />. It was discovered that when mice were SCD1-deficient, there was no obesity seen in the mice <ref name="Bai" /> This is why SCD1 is a popular target in treating metabolic diseases. Functioning SCD1 creates the balance between the accumulation and use of fats in the body. |
SCD-1 is interacts with either of the two different substrates: [https://pubchem.ncbi.nlm.nih.gov/compound/94140 stearoyl-CoA] or [https://pubchem.ncbi.nlm.nih.gov/compound/644109 palmitoyl-CoA]. When SCD1 interacts with stearoyl-CoA and performs desaturation, the product is [https://pubchem.ncbi.nlm.nih.gov/compound/Oleoyl-CoA oleoyl-CoA]and has the first cis-double bond introduced into the fatty acid chain. The introduction of the cis-double bond into the hydrocarbon chain will increase fluidity of the lipid bilayer. The process of desaturation is tightly regulated by multiple transcription factors. | SCD-1 is interacts with either of the two different substrates: [https://pubchem.ncbi.nlm.nih.gov/compound/94140 stearoyl-CoA] or [https://pubchem.ncbi.nlm.nih.gov/compound/644109 palmitoyl-CoA]. When SCD1 interacts with stearoyl-CoA and performs desaturation, the product is [https://pubchem.ncbi.nlm.nih.gov/compound/Oleoyl-CoA oleoyl-CoA]and has the first cis-double bond introduced into the fatty acid chain. The introduction of the cis-double bond into the hydrocarbon chain will increase fluidity of the lipid bilayer. The process of desaturation is tightly regulated by multiple transcription factors. | ||
| Line 37: | Line 37: | ||
====Product Conversion and Release==== | ====Product Conversion and Release==== | ||
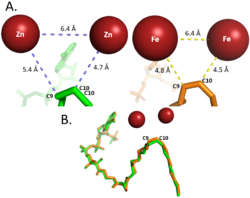
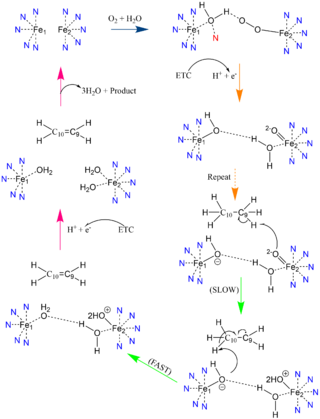
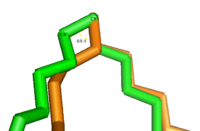
[[Image: Better_Gauch_vs._Eclipse.png|200 px|left|thumb|'''Change in conformation from reactant to product.''' The green substrate represents the conformation of the reactant and the orange substrate represents the conformation of the product. Substrates are stacked on top of each other in Fischer projection.]]The acyl-CoA substrate, due to it containing all sigma bonds, is able to freely rotate around its carbon axes to fit into the deep, kinked binding pocket.Upon double bond formation between C9 and C10, the substrate rotates from Gauche conformation to eclipsed conformation across C9 and C10 (Figure).The double bond also restricts rotation along carbons 9 and 10 which likely prevents the substrate from exiting through the site that it entered. To allow for <scene name='87/877510/Exit_of_substrate/3'>product exit</scene>, it is hypothesized that a hydrogen bond between Gln143 and Thr257 is broken which creates a hole below the kink into the hydrophobic core of the enzyme. This would allow for the lateral transfer of the product out of the binding pocket without removing the double bond. | [[Image: Better_Gauch_vs._Eclipse.png|200 px|left|thumb|'''Change in conformation from reactant to product.''' The green substrate represents the conformation of the reactant and the orange substrate represents the conformation of the product. Substrates are stacked on top of each other in Fischer projection.]]The acyl-CoA substrate, due to it containing all sigma bonds, is able to freely rotate around its carbon axes to fit into the deep, kinked binding pocket.Upon double bond formation between C9 and C10, the substrate rotates from Gauche conformation to eclipsed conformation across C9 and C10 (Figure).The double bond also restricts rotation along carbons 9 and 10 which likely prevents the substrate from exiting through the site that it entered. To allow for <scene name='87/877510/Exit_of_substrate/3'>product exit</scene>, it is hypothesized that a hydrogen bond between Gln143 and Thr257 is broken which creates a hole below the kink into the hydrophobic core of the enzyme. This would allow for the lateral transfer of the product out of the binding pocket without removing the double bond. | ||
| - | |||
Revision as of 16:09, 26 April 2021
Desaturation of Fatty Stearoyl-CoA by SCD1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers (Basel). 2019 Jul 5;11(7). pii: cancers11070948. doi:, 10.3390/cancers11070948. PMID:31284458 doi:http://dx.doi.org/10.3390/cancers11070948
- ↑ 3.0 3.1 3.2 Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
- ↑ Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, Li K, Sang BC. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat Struct Mol Biol. 2015 Jul;22(7):581-5. doi: 10.1038/nsmb.3049. Epub 2015 Jun , 22. PMID:26098317 doi:http://dx.doi.org/10.1038/nsmb.3049
- ↑ 5.0 5.1 Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006 Jun;116(6):1686-95. doi: 10.1172/JCI26991. PMID:16741579 doi:http://dx.doi.org/10.1172/JCI26991
- ↑ Yokoyama S, Hosoi T, Ozawa K. Stearoyl-CoA Desaturase 1 (SCD1) is a key factor mediating diabetes in MyD88-deficient mice. Gene. 2012 Apr 15;497(2):340-3. doi: 10.1016/j.gene.2012.01.024. Epub 2012 Feb 3. PMID:22326531 doi:http://dx.doi.org/10.1016/j.gene.2012.01.024
- ↑ Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11482-6. doi:, 10.1073/pnas.132384699. Epub 2002 Aug 12. PMID:12177411 doi:http://dx.doi.org/10.1073/pnas.132384699
- ↑ Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do KA, Fraser Symmans W, Pusztai L, Hortobagyi GN, Mills GB, Meric-Bernstam F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat. 2013 Jan;137(1):319-27. doi: 10.1007/s10549-012-2354-4. , Epub 2012 Dec 4. PMID:23208590 doi:http://dx.doi.org/10.1007/s10549-012-2354-4
- ↑ Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017 Mar 2;20(3):303-314.e5. doi: 10.1016/j.stem.2016.11.004., Epub 2016 Dec 29. PMID:28041894 doi:http://dx.doi.org/10.1016/j.stem.2016.11.004
Student Contributors
- Brianna M. Avery
- William J. Harris III
- Emily M. Royston