We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Kaitlyn Roberts/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
=== Active Site === | === Active Site === | ||
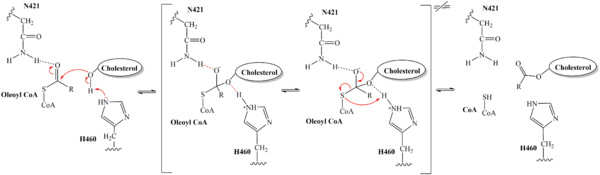
| - | + | Within the binding pocket, residues <scene name='87/877559/Important_residues/1'>W420, N421, H460</scene> serve as the key catalytic residues of SOAT, while various other residues aid in substrate binding and enzyme function.<ref name="Guan" /> Histidine, commonly used as the catalytic base for many acyl transferase reactions, is assumed to be the most important catalytic residue as it initiates the entire reaction. In the analysis of SOAT, this was confirmed as mutating H460 to alanine completely abolished enzymatic activity. <ref name="Qian" /> Additionally, H460 is highly conserved across a variety of species, further emphasizing its importance in SOAT catalysis.<ref name="Guan" /> It was hypothesized that N421 is responsible for stabilizing the transition state via an oxyanion hole with coenzymeA.<ref name="Qian" /> Additionally, mutations of W420A rendered the SOAT enzyme nonfunctional, indicating that it must be essential for catalytic activity. However, its role in the mechanism was not explicitly hypothesized. We believe that it plays a role in substrate binding through <scene name='87/879459/W420_intx/1'>hydrophobic interactions</scene> with CoenzymeA. SOAT activity also relies on several other <scene name='87/877559/Important_residues_2/1'>highly conserved residues</scene> within the interior of the central cavitity. This high preservation of residues suggests that the local environment plays a major role in SOAT activity. | |
=== Catalytic Mechanism === | === Catalytic Mechanism === | ||
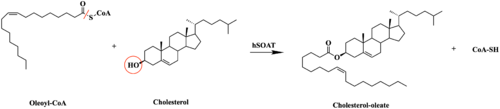
| - | The distal-most nitrogen on H460 acts as a base catalyst to deprotonate the hydroxyl group of | + | The substrate of interest, <scene name='87/877555/Oleoyl-coa_in_bp/1'>Oleoyl-CoA</scene>, is shown bound to SOAT to visualize the binding pocket. It must be noted that cholesterol, the other substrate involved, was never correctly imaged in the active site of SOAT. Upon binding of oleoyl-CoA and cholesterol to the SOAT active site, the distal-most nitrogen on H460 acts as a base catalyst to deprotonate the hydroxyl group of cholesterol. This leaves the cholesterol oxygen with a negative charge, making it a good [https://en.wikipedia.org/wiki/Nucleophile nucleophile]. The nucleophilic oxygen then attacks oleoyl-CoA at its carbonyl carbon, kicking electron density up to the carbonyl oxygen. The transition state is stabilized by <scene name='87/879459/As_acylcoa_interaction/2'>hydrogen bonding from N421</scene> and the newly protonated H460. [[Image:6p2pMechanism.png|600 px|right|thumb|'''Figure 5.''' Mechanism for the esterification reaction of SOAT with arrow pushing.]] |
From the transition state, excess electron density on the carbonyl oxygen is collapsed back into a double bond. This causes the bond between the carbonyl carbon and sulfur to break, shifting electron density to the sulfur atom. To complete the mechanism, the negatively charged sulfur would reclaim the hydrogen from protonated H460. Acyl CoA would exit the active site as a [https://en.wikipedia.org/wiki/Leaving_group leaving group], leaving its R group attached to cholesterol in the form of a cholesterol ester. | From the transition state, excess electron density on the carbonyl oxygen is collapsed back into a double bond. This causes the bond between the carbonyl carbon and sulfur to break, shifting electron density to the sulfur atom. To complete the mechanism, the negatively charged sulfur would reclaim the hydrogen from protonated H460. Acyl CoA would exit the active site as a [https://en.wikipedia.org/wiki/Leaving_group leaving group], leaving its R group attached to cholesterol in the form of a cholesterol ester. | ||
| - | It should be noted that this mechanism is largely hypothesized. Further analysis is needed to confirm the proposed steps | + | It should be noted that this mechanism is largely hypothesized. Further analysis is needed to confirm the proposed steps. |
== Inhibitors == | == Inhibitors == | ||
Revision as of 19:55, 26 April 2021
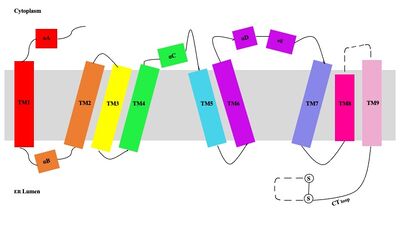
Human Sterol O-acyltransferase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 2.0 2.1 2.2 2.3 2.4 Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ 3.0 3.1 Bhattacharyya R, Kovacs DM. ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta. 2010 Aug;1801(8):960-5. doi: 10.1016/j.bbalip.2010.04.003. , Epub 2010 Apr 14. PMID:20398792 doi:http://dx.doi.org/10.1016/j.bbalip.2010.04.003
- ↑ 4.0 4.1 Huttunen HJ, Kovacs DM. ACAT as a drug target for Alzheimer's disease. Neurodegener Dis. 2008;5(3-4):212-4. doi: 10.1159/000113705. Epub 2008 Mar 6. PMID:18322393 doi:http://dx.doi.org/10.1159/000113705
- ↑ Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2006 Mar;38(3):151-6. doi:, 10.1111/j.1745-7270.2006.00154.x. PMID:16518538 doi:http://dx.doi.org/10.1111/j.1745-7270.2006.00154.x
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
Student Contributors
- Kylie Pfeifer
- Stephanie Pellegrino
- Kaitlyn Roberts