User:Brianna Avery/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
[https://en.wikipedia.org/wiki/Stearoyl-CoA_desaturase-1 Stearoyl-CoA Desaturase-1] <scene name='87/877504/New_main_structure/2'>(SCD1)</scene> is an integral membrane protein located in the endoplasmic reticulum and is conserved across all eukaryotes <ref name="Bai">DOI: 10.1038/nature14549</ref>. The SCD-1 pictured is expressed in Mus musculus (house mouse). The human homolog, SCD1, shares 85% sequence identity with all four SCD’s found in M. musculus (SCD1-SCD4). The expression of SCD1 is seen mainly in the liver and brain <ref name="Dobrzyn">PMID: 31284458</ref>. | [https://en.wikipedia.org/wiki/Stearoyl-CoA_desaturase-1 Stearoyl-CoA Desaturase-1] <scene name='87/877504/New_main_structure/2'>(SCD1)</scene> is an integral membrane protein located in the endoplasmic reticulum and is conserved across all eukaryotes <ref name="Bai">DOI: 10.1038/nature14549</ref>. The SCD-1 pictured is expressed in Mus musculus (house mouse). The human homolog, SCD1, shares 85% sequence identity with all four SCD’s found in M. musculus (SCD1-SCD4). The expression of SCD1 is seen mainly in the liver and brain <ref name="Dobrzyn">PMID: 31284458</ref>. | ||
| - | SCD is an enzyme which catalyzes desaturation of a double bond within a fatty acid hydrocarbon chain. The addition of a double bond is necessary for the biosynthesis of monounsaturated fatty acids such as: cholesterol, phospholipids, and triglycerides. The enzyme’s main function is [https://en.wikipedia.org/wiki/Lipid_metabolism#:~:text=%2B-,Lipid%20biosynthesis,the%20organisms%20through%20various%20pathways. lipid biosynthesis] as well as regulating gene expression for [https://en.wikipedia.org/wiki/Lipogenesis#:~:text=Lipogenesis%20is%20the%20metabolic%20process,packaged%20within%20cytoplasmic%20lipid%20droplets. lipogensis] <ref name="Bai" />. SCD1 is regulated by transcription and its promoter has multiple binding sites for transcription factors that assist with regulation of lipogenesis <ref name="Dobrzyn" />. It was discovered that when mice were SCD1-deficient, there was no obesity seen in the mice <ref name="Bai" /> This is why SCD1 is a popular target in treating metabolic diseases. Functioning SCD1 creates the balance between the accumulation and use of fats in the body. [[Image:Screen_Shot_2021-04-26_at_3.24.49_PM.png|250 px|left|thumb|Saturated vs. Unsaturated Fatty Acid Chain]] | + | SCD is an enzyme which catalyzes desaturation of a double bond within a fatty acid hydrocarbon chain. The addition of a double bond is necessary for the biosynthesis of monounsaturated fatty acids such as: cholesterol, phospholipids, and triglycerides. The enzyme’s main function is [https://en.wikipedia.org/wiki/Lipid_metabolism#:~:text=%2B-,Lipid%20biosynthesis,the%20organisms%20through%20various%20pathways. lipid biosynthesis] as well as regulating gene expression for [https://en.wikipedia.org/wiki/Lipogenesis#:~:text=Lipogenesis%20is%20the%20metabolic%20process,packaged%20within%20cytoplasmic%20lipid%20droplets. lipogensis] <ref name="Bai" />. SCD1 is regulated by transcription and its promoter has multiple binding sites for transcription factors that assist with regulation of lipogenesis <ref name="Dobrzyn" />. It was discovered that when mice were SCD1-deficient, there was no obesity seen in the mice <ref name="Bai" /> This is why SCD1 is a popular target in treating metabolic diseases. Functioning SCD1 creates the balance between the accumulation and use of fats in the body. [[Image:Screen_Shot_2021-04-26_at_3.24.49_PM.png|250 px|left|thumb|'''Figure 1: Saturated vs. Unsaturated Fatty Acid Chain.''']] |
SCD-1 is interacts with either of the two different substrates: [https://pubchem.ncbi.nlm.nih.gov/compound/94140 stearoyl-CoA] or [https://pubchem.ncbi.nlm.nih.gov/compound/644109 palmitoyl-CoA]. When SCD1 interacts with stearoyl-CoA and performs desaturation, the product is [https://pubchem.ncbi.nlm.nih.gov/compound/Oleoyl-CoA oleoyl-CoA]and has the first cis-double bond introduced into the fatty acid chain. The introduction of the cis-double bond into the hydrocarbon chain will increase fluidity of the lipid bilayer. The process of desaturation is tightly regulated by multiple transcription factors. | SCD-1 is interacts with either of the two different substrates: [https://pubchem.ncbi.nlm.nih.gov/compound/94140 stearoyl-CoA] or [https://pubchem.ncbi.nlm.nih.gov/compound/644109 palmitoyl-CoA]. When SCD1 interacts with stearoyl-CoA and performs desaturation, the product is [https://pubchem.ncbi.nlm.nih.gov/compound/Oleoyl-CoA oleoyl-CoA]and has the first cis-double bond introduced into the fatty acid chain. The introduction of the cis-double bond into the hydrocarbon chain will increase fluidity of the lipid bilayer. The process of desaturation is tightly regulated by multiple transcription factors. | ||
| Line 21: | Line 21: | ||
====Metal Cations==== | ====Metal Cations==== | ||
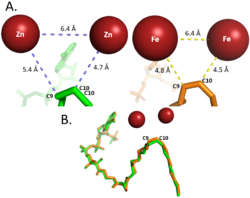
| - | [[Image:Use_Metal_Cation_Figure.png|250 px|right|thumb|'''Differences between zinc and iron di-metal interactions with acetyl-CoA.''']]Typically, SCD1 contains a <scene name='87/877504/Di_metal_center/3'>di-metal center</scene> in the core of the protein that contributes to its desaturation function. Crystallized structures of SCD1 indicated a substitution of Zinc as the di-metal center instead of <ref name="Bai" />. It is proposed that the difference in size of Zinc and Iron (8.8 Å and 9.2 Å respectively) would not affect the structure of SCD. However, the substitution of Zinc as the di-iron center is found to inhibit the function of SCD1 due to a farther distance between the two metals compared to iron (Figure containing all 3 of Treys photos) <ref name="Bai" />. | + | [[Image:Use_Metal_Cation_Figure.png|250 px|right|thumb|'''Figure 2: Differences between zinc and iron di-metal interactions with acetyl-CoA.''']]Typically, SCD1 contains a <scene name='87/877504/Di_metal_center/3'>di-metal center</scene> in the core of the protein that contributes to its desaturation function. Crystallized structures of SCD1 indicated a substitution of Zinc as the di-metal center instead of <ref name="Bai" />. It is proposed that the difference in size of Zinc and Iron (8.8 Å and 9.2 Å respectively) would not affect the structure of SCD. However, the substitution of Zinc as the di-iron center is found to inhibit the function of SCD1 due to a farther distance between the two metals compared to iron (Figure containing all 3 of Treys photos) <ref name="Bai" />. |
In other di-metal centered enzymes such as [https://en.wikipedia.org/wiki/Acyl-(acyl-carrier-protein)_desaturase ACP desaturase] and [https://en.wikipedia.org/wiki/Ribonucleotide_reductase ribonucleotide reductase], the enzymatic mechanism involves an oxo-bridge: a water molecule that is recruited by the di-iron center to be directly involved in the desaturase mechanism. This water gets deprotonated by the two metal ions and it becomes nucleophilic enough to attack the substrate (Mechanism figure). Based on electron density mapping of SCD1, this oxo-bridge formation is suggested to be short-lived <ref name="Shen">DOI: 10.1016/j.jmb.2020.05.017</ref>. | In other di-metal centered enzymes such as [https://en.wikipedia.org/wiki/Acyl-(acyl-carrier-protein)_desaturase ACP desaturase] and [https://en.wikipedia.org/wiki/Ribonucleotide_reductase ribonucleotide reductase], the enzymatic mechanism involves an oxo-bridge: a water molecule that is recruited by the di-iron center to be directly involved in the desaturase mechanism. This water gets deprotonated by the two metal ions and it becomes nucleophilic enough to attack the substrate (Mechanism figure). Based on electron density mapping of SCD1, this oxo-bridge formation is suggested to be short-lived <ref name="Shen">DOI: 10.1016/j.jmb.2020.05.017</ref>. | ||
| Line 33: | Line 33: | ||
====Desaturation Mechanism==== | ====Desaturation Mechanism==== | ||
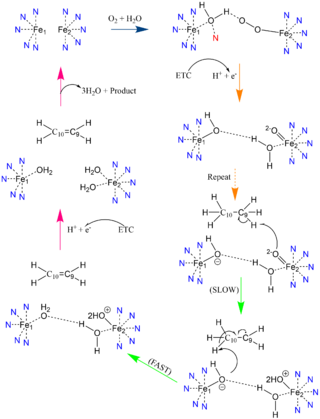
| - | [[Image:Better_SCD_mech_pic.png|320 px|right|thumb|Proposed mechanism of SCD1 based off Shen et al. 2020 <ref name="Shen" />. Nitrogens colored blue represent the histidine stabilization of the di-iron complex while the red nitrogen is the water-stabilizing asparagine.]]Due to the novel coordination of histidine residues and lack of presence of a stable oxo-bridge, the mechanism of desaturase activity for SCD1 is unknown. For typical desaturases, the elements involved in catalysis include the di-metal center, metal-bound water and oxygen, electrons from an Electron Transport Chain, and the acyl-CoA substrate. For the proposed mechanism, catalysis occurs in four phases: the binding of water and oxygen to the di-metal complex using Asn261 (blue arrow), the nucleophilic activation of the water/oxygen using the ETC (orange arrows), the interaction of the complex with the substrate (green arrows), and the recovery of the enzyme (pink arrows). The rate-limiting step of the mechanism is the primary elimination reaction on the substrate due to the nucleophilic attack occurring on an already stable C9-C10 sigma bond. | + | [[Image:Better_SCD_mech_pic.png|320 px|right|thumb|'''Figure 3: Proposed mechanism of SCD1 based off Shen et al. 2020''' <ref name="Shen" />. Nitrogens colored blue represent the histidine stabilization of the di-iron complex while the red nitrogen is the water-stabilizing asparagine.]]Due to the novel coordination of histidine residues and lack of presence of a stable oxo-bridge, the mechanism of desaturase activity for SCD1 is unknown. For typical desaturases, the elements involved in catalysis include the di-metal center, metal-bound water and oxygen, electrons from an Electron Transport Chain, and the acyl-CoA substrate. For the proposed mechanism, catalysis occurs in four phases: the binding of water and oxygen to the di-metal complex using Asn261 (blue arrow), the nucleophilic activation of the water/oxygen using the ETC (orange arrows), the interaction of the complex with the substrate (green arrows), and the recovery of the enzyme (pink arrows). The rate-limiting step of the mechanism is the primary elimination reaction on the substrate due to the nucleophilic attack occurring on an already stable C9-C10 sigma bond. |
====Product Conversion and Release==== | ====Product Conversion and Release==== | ||
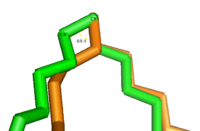
| - | [[Image: Better_Gauch_vs._Eclipse.png|200 px|left|thumb|'''Change in conformation from reactant to product.''' The green substrate represents the conformation of the reactant and the orange substrate represents the conformation of the product. Substrates are stacked on top of each other in Fischer projection.]]The acyl-CoA substrate, due to it containing all sigma bonds, is able to freely rotate around its carbon axes to fit into the deep, kinked binding pocket.Upon double bond formation between C9 and C10, the substrate rotates from Gauche conformation to eclipsed conformation across C9 and C10 (Figure).The double bond also restricts rotation along carbons 9 and 10 which likely prevents the substrate from exiting through the site that it entered. To allow for <scene name='87/877504/Exit_of_substrate_1/6'>product exit</scene>, it is hypothesized that a hydrogen bond between Gln143 and Thr257 is broken which creates a hole below the kink into the hydrophobic core of the enzyme. This would allow for the lateral transfer of the product out of the binding pocket without removing the double bond. | + | [[Image: Better_Gauch_vs._Eclipse.png|200 px|left|thumb|'''Figure 4: Change in conformation from reactant to product.''' The green substrate represents the conformation of the reactant and the orange substrate represents the conformation of the product. Substrates are stacked on top of each other in Fischer projection.]]The acyl-CoA substrate, due to it containing all sigma bonds, is able to freely rotate around its carbon axes to fit into the deep, kinked binding pocket.Upon double bond formation between C9 and C10, the substrate rotates from Gauche conformation to eclipsed conformation across C9 and C10 (Figure).The double bond also restricts rotation along carbons 9 and 10 which likely prevents the substrate from exiting through the site that it entered. To allow for <scene name='87/877504/Exit_of_substrate_1/6'>product exit</scene>, it is hypothesized that a hydrogen bond between Gln143 and Thr257 is broken which creates a hole below the kink into the hydrophobic core of the enzyme. This would allow for the lateral transfer of the product out of the binding pocket without removing the double bond. |
Revision as of 19:33, 26 April 2021
Desaturation of Fatty Stearoyl-CoA by SCD1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers (Basel). 2019 Jul 5;11(7). pii: cancers11070948. doi:, 10.3390/cancers11070948. PMID:31284458 doi:http://dx.doi.org/10.3390/cancers11070948
- ↑ 3.0 3.1 3.2 Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
- ↑ Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, Li K, Sang BC. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat Struct Mol Biol. 2015 Jul;22(7):581-5. doi: 10.1038/nsmb.3049. Epub 2015 Jun , 22. PMID:26098317 doi:http://dx.doi.org/10.1038/nsmb.3049
- ↑ 5.0 5.1 Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006 Jun;116(6):1686-95. doi: 10.1172/JCI26991. PMID:16741579 doi:http://dx.doi.org/10.1172/JCI26991
- ↑ Yokoyama S, Hosoi T, Ozawa K. Stearoyl-CoA Desaturase 1 (SCD1) is a key factor mediating diabetes in MyD88-deficient mice. Gene. 2012 Apr 15;497(2):340-3. doi: 10.1016/j.gene.2012.01.024. Epub 2012 Feb 3. PMID:22326531 doi:http://dx.doi.org/10.1016/j.gene.2012.01.024
- ↑ Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11482-6. doi:, 10.1073/pnas.132384699. Epub 2002 Aug 12. PMID:12177411 doi:http://dx.doi.org/10.1073/pnas.132384699
- ↑ Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do KA, Fraser Symmans W, Pusztai L, Hortobagyi GN, Mills GB, Meric-Bernstam F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat. 2013 Jan;137(1):319-27. doi: 10.1007/s10549-012-2354-4. , Epub 2012 Dec 4. PMID:23208590 doi:http://dx.doi.org/10.1007/s10549-012-2354-4
- ↑ Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017 Mar 2;20(3):303-314.e5. doi: 10.1016/j.stem.2016.11.004., Epub 2016 Dec 29. PMID:28041894 doi:http://dx.doi.org/10.1016/j.stem.2016.11.004
Student Contributors
- Brianna M. Avery
- William J. Harris III
- Emily M. Royston