We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jacob Holt/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
=== Ligand Binding Pocket and Histidine Coordination === | === Ligand Binding Pocket and Histidine Coordination === | ||
| - | The ligand binding pocket is a narrow tunnel that extends approximately 24 Å into the mostly hydrophobic interior of the protein. The ligand is stabilized by bending into a kinked conformation which creates a tight fit in the binding pocket tunnel, and by a hydrogen bond that occurs between the <scene name='87/877552/W258/ | + | The ligand binding pocket is a narrow tunnel that extends approximately 24 Å into the mostly hydrophobic interior of the protein. The ligand is stabilized by bending into a kinked conformation which creates a tight fit in the binding pocket tunnel, and by a hydrogen bond that occurs between the <scene name='87/877552/W258/4'>W 258</scene> side chain and the acyl carbonyl<ref name="Bai" />. The kink in the tunnel is formed by the conserved residues, <scene name='87/877552/Desaturation_site/10'>T 257 and W 149</scene> which are stabilized by the hydrogen bond shared with Q143<ref name="Bai" />. There are <scene name='87/877552/Substrate_orientation_w_fe/7'>two Fe2+ ions</scene> that interact with the substrate; the Fe2+ ions are coordinated by <scene name='87/877552/Histidine_coordination/8'>9 invariant histidine residues</scene>. <scene name='87/877552/Substrate_oreintation_fe_90deg/4'>When rotated 90 degrees</scene> the ligand is seen to be in a eclipsed position, indicating it is in its post-reaction form. One metal ion is coordinated by 4 histidines residues and a water molecule, and the other metal ion is coordinated by 5 histidine residues<ref name="Bai" />. The histidine residues position the metal ions 6.4 Å apart<ref name="Bai" />. |
=== Desaturation Site === | === Desaturation Site === | ||
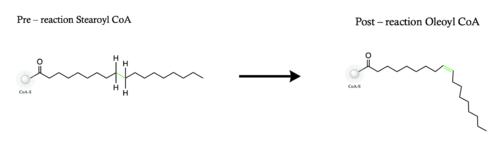
| - | The ligand is desaturated at carbons 9 and 10<ref name="Shen" />. The desaturation site of the ligand takes place inside the active site tunnel which enforces correct positioning of the substrate<ref name="Bai" />. Before the reaction occurs, the ligand is in a gauche conformation at the desaturation site. This was determined by accidental usage of <scene name='87/877552/Pre_reaction_substrate_zn/ | + | The ligand is desaturated at carbons 9 and 10<ref name="Shen" />. The desaturation site of the ligand takes place inside the active site tunnel which enforces correct positioning of the substrate<ref name="Bai" />. Before the reaction occurs, the ligand is in a gauche conformation at the desaturation site. This was determined by accidental usage of <scene name='87/877552/Pre_reaction_substrate_zn/3'>Zn+ ions</scene> which allowed for binding of the substrate but prevented the reaction<ref name="Shen" />. The product is in a cis conformation post-reaction. The product structure was determined using Fe2+ metal ions which allowed for the full reaction to take place<ref name="Shen" />. The difference between the <scene name='87/877552/Overlay_of_ligands/6'>substrate and product</scene> is the creation of a double bond, and the positioning of carbon 9 and 10 into a eclipsed position |
=== Active Site Cap === | === Active Site Cap === | ||
| - | The two conserved residues of the active site cap are <scene name='87/877552/Active_site_cap/ | + | The two conserved residues of the active site cap are <scene name='87/877552/Active_site_cap/8'>Y 104 and G 287</scene>. These two residues form a hydrogen bond creating a rigid barrier at the end of the active site to keep the ligand from moving during the reaction<ref name="Bai" />. The active site cap is also used in determining the substrate length when entering the active site<ref name="Bai" />. |
=== Catalytic Molecule === | === Catalytic Molecule === | ||
| - | The <scene name='87/877552/Water/ | + | The <scene name='87/877552/Water/4'>catalytic molecule</scene> of the SCD1 enzyme is a water molecule, coordinated by <scene name='87/877552/Asparagine_h20_stabilization/3'>N 261</scene> via hydrogen bonding<ref name="Bai" />. The water molecule is 2.2 Å away from the Fe2+ metal ion molecule<ref name="Bai" />. It interacts with the Fe2+ ion to make highly reactive radicals that are able to desaturate the highly stable carbon chain<ref name="Yu">DOI:10.1021/acscatal.9b00456 </ref>. It is through the coordination of these ions by the histidines that the <scene name='87/877552/Diiron_center/9'>substrate and catalytic molecule</scene> are able to be positioned within the vicinity of carbons 9 and 10 of the ligand<ref name="Yu" />. |
=== Substrate Entering and Leaving === | === Substrate Entering and Leaving === | ||
| - | The substrate enters the active site through the active site tunnel and undergoes a <scene name='87/877552/Substrate_entry_exit/ | + | The substrate enters the active site through the active site tunnel and undergoes a <scene name='87/877552/Substrate_entry_exit/4'>conformational change</scene> to conform to the kinked shape of the tunnel<ref name="Bai" />. Upon the substrate being converted to its final form, the product is laterally released from the protein due to a lateral egress of H1 and H2 caused by the deformation in the hydrogen bonding of the residues N 143 and T 257<ref name="Bai" />. |
| Line 70: | Line 70: | ||
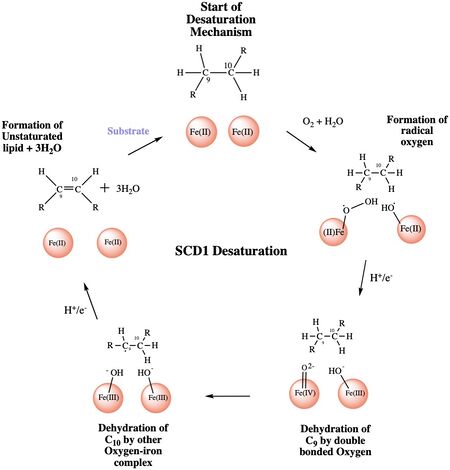
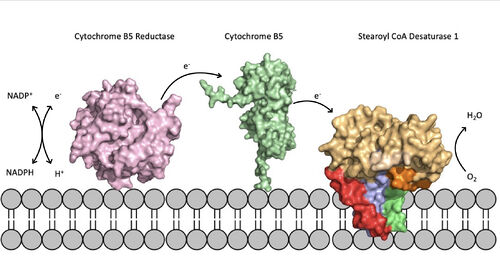
'''Step 1''': Addition of O2 and H2O which react with the Fe2+ ions to create oxygen radicals on the iron ions (Figure 4). | '''Step 1''': Addition of O2 and H2O which react with the Fe2+ ions to create oxygen radicals on the iron ions (Figure 4). | ||
| - | '''Step 2''': Electron/proton pair is brought in via the electron transport chain; this increases the oxidation of both iron ions, gets rid of the radicals, and creates an active Fe-oxyl molecule (Figure). | + | '''Step 2''': Electron/proton pair is brought in via the electron transport chain; this increases the oxidation of both iron ions, gets rid of the radicals, and creates an active Fe-oxyl molecule (Figure)<ref name="Yu" />. Fe-oxyl molecule is reactive enough, due to the change in the oxidation state, to pull off the first hydrogen on carbon 9 (Figure 4). |
| - | '''Step 3''': An unstable radical intermediate of the 18-carbon acyl-CoA ligand is formed which reacts with the other Fe-O molecule, in the +3 state, to pull of the second hydrogen and form the final product ( | + | '''Step 3''': An unstable radical intermediate of the 18-carbon acyl-CoA ligand is formed which reacts with the other Fe-O molecule, in the +3 state, to pull of the second hydrogen and form the final product (Figure 4)<ref name="Yu" />. |
'''Step 4''': Another electron/proton pair is brought in to create three H2O molecules and to take the Fe ions back down to their original oxidation state of +2 (Figure 4)<ref name="Yu" />. | '''Step 4''': Another electron/proton pair is brought in to create three H2O molecules and to take the Fe ions back down to their original oxidation state of +2 (Figure 4)<ref name="Yu" />. | ||
Revision as of 14:45, 27 April 2021
Desaturation of Fatty Acids using Stearoyl-CoA Desaturase-1 Enzyme
| |||||||||||
Student Contributions
Carson Maris, Jess Kersey, Jacob Holt