We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Sarah Maarouf/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 122: | Line 122: | ||
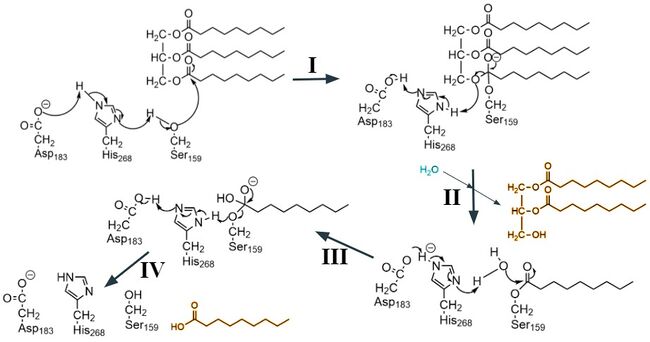
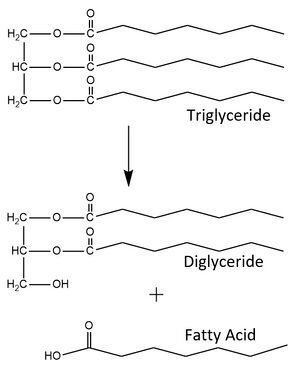
Missense mutations in LPL or GPIHBP1 can lead to [https://en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia], a condition characterized by high levels of triglycerides in the blood. Two specific point mutations that contribute to this disease through the abolishment of LPL-GPIHBP1 binding are <scene name='87/877554/Met404_cys445/4'>M404R and C445Y</scene>. Because the LPL-GPIHBP1 complex is bound together primarily by hydrophobic interactions, switching a nonpolar [https://en.wikipedia.org/wiki/Methionine methionine] residue to a positively charged [https://en.wikipedia.org/wiki/Arginine arginine] residue, or a nonpolar [https://en.wikipedia.org/wiki/Cysteine cysteine] residue to a polar [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residue will disrupt these hydrophobic interactions and weaken the interface. Also, the much larger side chain of arginine is difficult to fit into the hydrophobic pocket of GPIHBP1. <ref name="Birrane">PMID:30559189</ref> These mutations do not affect LPL’s activity or secretion but play a role its ability to be transported to its site of action in the capillary lumen due to the inability of GPIHBP1 to bind and transport LPL. This leads to an accumulation of unmetabolized lipids but also catalytically active LPL in the interstitial spaces.<ref name="Voss">PMID:21518912</ref> | Missense mutations in LPL or GPIHBP1 can lead to [https://en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia], a condition characterized by high levels of triglycerides in the blood. Two specific point mutations that contribute to this disease through the abolishment of LPL-GPIHBP1 binding are <scene name='87/877554/Met404_cys445/4'>M404R and C445Y</scene>. Because the LPL-GPIHBP1 complex is bound together primarily by hydrophobic interactions, switching a nonpolar [https://en.wikipedia.org/wiki/Methionine methionine] residue to a positively charged [https://en.wikipedia.org/wiki/Arginine arginine] residue, or a nonpolar [https://en.wikipedia.org/wiki/Cysteine cysteine] residue to a polar [https://en.wikipedia.org/wiki/Tyrosine tyrosine] residue will disrupt these hydrophobic interactions and weaken the interface. Also, the much larger side chain of arginine is difficult to fit into the hydrophobic pocket of GPIHBP1. <ref name="Birrane">PMID:30559189</ref> These mutations do not affect LPL’s activity or secretion but play a role its ability to be transported to its site of action in the capillary lumen due to the inability of GPIHBP1 to bind and transport LPL. This leads to an accumulation of unmetabolized lipids but also catalytically active LPL in the interstitial spaces.<ref name="Voss">PMID:21518912</ref> | ||
| - | Additionally, the carboxylic acid side chain of D201 is critical for coordinating LPL’s calcium ion, and the point mutations <scene name='87/877554/Mutation_of_d202_and_d201/12'>D201V and D202E</scene> found in patients with [https://medlineplus.gov/ency/article/000405.htm#:~:text=Chylomicronemia%20syndrome%20is%20a%20disorder,is%20passed%20down%20through%20families. chylomicronemia] have been observed to eliminate LPL secretion and reduce its activity.<ref name="Young">PMID:31269429</ref> Mutating this negatively charged [https://en.wikipedia.org/wiki/Aspartic_acid aspartic acid] into a nonpolar [https://en.wikipedia.org/wiki/Valine valine] residue disrupts the ionic bond between calcium and the aspartate, disturbing the overall calcium binding. A similar mutation has also been observed for D202E. These two mutations, in turn, destabilize LPL folding and thereby prevent its secretion from cells.<ref name="Birrane">PMID:30559189</ref> Knowledge about this enzyme's structure, function, and mutations will help create potential therapeutics to treat chylomicronemia and hypertriglyceridemia | + | Additionally, the carboxylic acid side chain of D201 is critical for coordinating LPL’s calcium ion, and the point mutations <scene name='87/877554/Mutation_of_d202_and_d201/12'>D201V and D202E</scene> found in patients with [https://medlineplus.gov/ency/article/000405.htm#:~:text=Chylomicronemia%20syndrome%20is%20a%20disorder,is%20passed%20down%20through%20families. chylomicronemia] have been observed to eliminate LPL secretion and reduce its activity.<ref name="Young">PMID:31269429</ref> Mutating this negatively charged [https://en.wikipedia.org/wiki/Aspartic_acid aspartic acid] into a nonpolar [https://en.wikipedia.org/wiki/Valine valine] residue disrupts the ionic bond between calcium and the aspartate, disturbing the overall calcium binding. A similar mutation has also been observed for D202E. These two mutations, in turn, destabilize LPL folding and thereby prevent its secretion from cells.<ref name="Birrane">PMID:30559189</ref> Knowledge about this enzyme's structure, function, and mutations will help create potential therapeutics to treat chylomicronemia and hypertriglyceridemia, reducing the risk of developing cardiovascular problems.<ref name="Arora">PMID:31072929</ref> |
Revision as of 14:25, 27 April 2021
H. sapiens Lipoprotein Lipase in complex with GPIHBP1 and triglyceride metabolism
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Fong LG, Young SG, Beigneux AP, Bensadoun A, Oberer M, Jiang H, Ploug M. GPIHBP1 and Plasma Triglyceride Metabolism. Trends Endocrinol Metab. 2016 Jul;27(7):455-469. doi: 10.1016/j.tem.2016.04.013. , Epub 2016 May 14. PMID:27185325 doi:http://dx.doi.org/10.1016/j.tem.2016.04.013
- ↑ 2.0 2.1 Voss CV, Davies BS, Tat S, Gin P, Fong LG, Pelletier C, Mottler CD, Bensadoun A, Beigneux AP, Young SG. Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7980-4. doi:, 10.1073/pnas.1100992108. Epub 2011 Apr 25. PMID:21518912 doi:http://dx.doi.org/10.1073/pnas.1100992108
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
- ↑ 4.0 4.1 Young SG, Fong LG, Beigneux AP, Allan CM, He C, Jiang H, Nakajima K, Meiyappan M, Birrane G, Ploug M. GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism. Cell Metab. 2019 Jul 2;30(1):51-65. doi: 10.1016/j.cmet.2019.05.023. PMID:31269429 doi:http://dx.doi.org/10.1016/j.cmet.2019.05.023
- ↑ Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol. 2016 Jun;27(3):233-41. doi: 10.1097/MOL.0000000000000297. PMID:27031275 doi:http://dx.doi.org/10.1097/MOL.0000000000000297
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ Hedstrom L. Serine Protease Mechanism and Specificity. Chemical Reviews 2002 102 (12), 4501-4524 https://doi.org/10.1021/cr000033x
Student Contributors
- Aniyah Coles
- Sarah Maarouf
- Audrey Marjamaa
![Figure 2. A cartoon representation of GPIHBP1's N-terminal intrinsically disordered region (IDR) colored green and an surface representation of LPL with electrostatic coloring [acidic (red), neutral (white), basic (blue)] to show how GPIHBP1's N-terminal domain interacts with LPL's basic patch to stabilize LPL structure and activity.](/wiki/images/thumb/7/72/Electro.jpg/250px-Electro.jpg)