Introduction
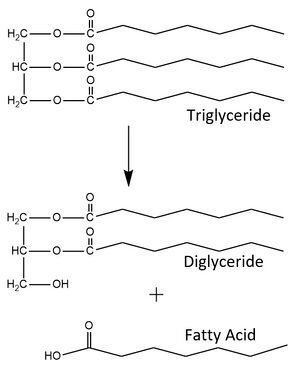
Figure 1. LPL catalyzes the breakdown of triglycerides into a diglyceride and a fatty acid. It can also recognize the diglyceride as a substrate and produce a monoglyceride and two fatty acids from the triglyceride substrate.
is an enzyme synthesized and secreted primarily by myocytes and adipocytes into interstitial spaces.[1] It is located on the surface of capillaries where it is bound to a glycolipid-anchored protein expressed by capillary endothelial cells. This protein is called glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1, or GPIHBP1.[2] LPL is an essential enzyme for triglyceride metabolism and utilization, however it is susceptible to unfolding in its catalytic domain, and thus must be bound to GPIHBP1 to prevent loss of enzymatic activity. When LPL is not bound to GPIHBP1 its enzymatic activity is relatively low and declines until it has lost all function. However, when bound to GPIHBP1 it is able to maintain its maximum enzymatic activity.[3] In addition, binding to GPIHBP1 is required for adhesion of triglyceride rich lipoproteins (TRLs) to LPL and transport of LPL to its site of action in the capillary lumen. Once it has reached the site of action, the enzyme is able to produce a monoglyceride and two fatty acids from the triglyceride substrate (Figure 1).[4]
Function
Energy is metabolized through the hydrolysis of triglyceride-rich lipoproteins (TRLs), among other macromolecules, to release fatty acids that can be used or stored for later energy use. [5] LPL is the main enzyme involved with the metabolism of triglycerides within TRLs. The LPL-GPIHBP1 complex is crucial for clearing triglycerides from the bloodstream and delivery of lipid nutrients to vital areas, such as heart and skeletal muscle, and adipose tissue.[6] Loss of function mutations in LPL or GPIHBP1 can have detrimental effects on the body's ability to metabolize triglycerides which in turn causes chylomicronemia. Elevated levels of plasma triglycerides, called hypertriglyceridemia, also increases the risk of developing coronary artery disease (CAD).[1]
Structure
The asymmetric unit is a of LPL-GPIHBP1 complexes. The orientation of these four dimers are in a head-to-tail conformation. Despite the unique orientation of each of the four dimers within the tetramer, all act independently and perform the same enzymatic function. Each complex consists of the LPL with N-terminal and C-terminal domains, and the associated GPIHBP1.[3]
N-terminal α/β-hydrolase domain of LPL
The is composed of one antiparallel β-sheet and seven parallel β-sheets that are located between five α-helices. This is the domain responsible for hydrolysis of lipid substrates, as it contains the and houses the to stabilize the transition state of the substrate. The N-terminal domain includes a which has been shown to have mutations that may impact LPL enzyme activity. The lid region of the N-terminal domain was imaged in an open conformation, meaning it is not blocking the active site. The consists of 2 short α-helices connected by a loop, extending away from the protein. This open conformation allows for many surface-exposed hydrophobic residues (valines, isoleucines, and leucines) to create a hydrophobic patch on the surface of LPL. The lid region helps to control for the entry of lipid substrates into the active site cleft, though specifics about how lipids enter into the active site still requires further investigation.[3]
C-terminal β-barrel domain of LPL
The of LPL is made up of 8 antiparallel β-sheets.[3] It includes the GPIHBP1 binding site and the that helps to contribute to the specificity of this enzyme for TRLs by creating a second hydrophobic patch on the same face of LPL as the active site and the lid region. The hydrophobic patches are believed to allow for the enzyme to bind TRL substrates with an orientation that facilitates delivery of triglycerides into the active site for catalysis. [6] The lid region and the lipid binding region mechanism of interaction and protein flexibility for binding TRL substrates still needs further investigation. [3]
GPIHBP1
![Figure 2. A cartoon representation of GPIHBP1's N-terminal intrinsically disordered region (IDR) colored green and an surface representation of LPL with electrostatic coloring [acidic (red), neutral (white), basic (blue)] to show how GPIHBP1's N-terminal domain interacts with LPL's basic patch to stabilize LPL structure and activity.](/wiki/images/thumb/7/72/Electro.jpg/250px-Electro.jpg)
Figure 2. A cartoon representation of GPIHBP1's N-terminal intrinsically disordered region (IDR) colored green and an surface representation of LPL with electrostatic coloring [acidic (red), neutral (white), basic (blue)] to show how GPIHBP1's N-terminal domain interacts with LPL's basic patch to stabilize LPL structure and activity.
GPIHBP1 is a membrane bound protein that binds to the C-terminal domain of LPL. It contains specific features, such as the cysteine-rich LU domain and an intrinsically disordered acidic N-terminal domain, that contribute to its binding affinity of LPL. GPIHBP1’s LU domain (Ly6-uPar) is 75 residues in length and adopts a , a characteristic of the LU protein family, that is stabilized by . The LPL-GPIHBP1 binding interface involves the three-fingered fold and depends largely on hydrophobic interactions between the two subunits which creates a high binding affinity of LPL. The disordered acidic N-terminal domain of GPIHBP1 plays a role in capturing LPL, also contributing to binding affinity.[1] The disordered N-terminal domain has 21 of the 26 total residues being aspartates or glutamates. Unfortunately images for the acidic domain were not resolved, indicating that it likely exhibits conformational flexibility.[3] Because GPIHBP1 makes electrostatic interactions with LPL's basic patch (Figure 2), this complex between GPIHBP1's acidic N-terminal domain and LPL's C-terminal domain is speculated to create the stability in LPL that allows for its catalytic activity to continue when bound to GPIHBP1.[6]
Active Site
by which this enzyme catalyzes the hydrolysis of triglycerides is located in the N-terminal domain of LPL, and the catalytic residues involved in this reaction are Ser159, Asp183, and His268.[6] Located near these residues, the stabilizes the transition state of the substrate through the backbone amides of Trp82 and Leu160.[3] Surrounding the active site is the which stabilizes the hydrophobic lipid substrates in the binding pocket and forms Van der Waals interactions with the hydrophobic tails of the lipids that are entering. This hydrophobic region also allows the substrates to access the for hydrolysis.[6]
Mechanism
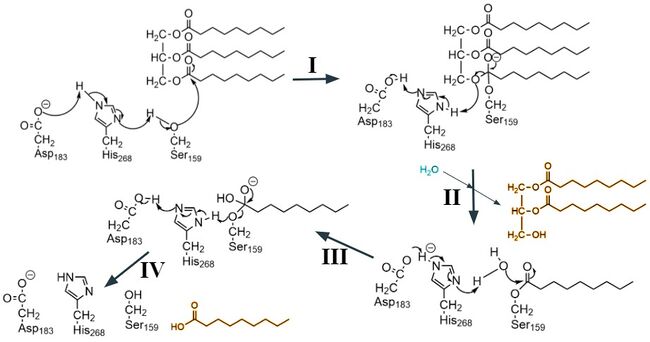
Figure 3. A four step arrow pushing mechanism for the catalysis of a triglyceride. The diglyceride and fatty acid products are highlighted in yellow. The water molecule that makes the second nucleophilic attack is highlighted in blue.
The active site consists of a catalytic triad Ser159, Asp183 and His268 that go through the serine protease mechanism of action. The hallmark of this mechanism is the proton shuttle between the three residues that increases the nucleophilicity of the serine residue. Serine is then able to make the nucleophilic attack on the the carbonyl carbon of the scissile peptide bond of the substrate (step I). There are several intermediates generated in this ordered mechanism. The first round of the catalysis is where the triglyceride binds, and the diglyceride is released (step II). Then the second substrate, water, binds (step III) and the second product, the fatty acid, is released (step IV).[7]
Inhibitor
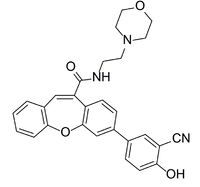
Figure 4. The structure of the inhibitor referred to as Compound 2.
The active site residues of LPL can be blocked from preforming the breakdown of triglycerides by the presence of a competitive . The inhibitor binds in the hydrophobic binding region and occludes access to the catalytic triad. The inhibitor known as Compound 2 (Figure 4) was designed to stabilize the structure of the LPL-GPIHBP1 complex for the purpose of structure determination.[3] Although the complex structure was resolved with two molecules of Compound 2 in the hydrophobic binding region, only the proximal inhibitor interacts with the catalytic triad.
Medical Relevance
Mutations
Missense mutations in LPL or GPIHBP1 can lead to hypertriglyceridemia, a condition characterized by high levels of triglycerides in the blood. Two specific point mutations that contribute to this disease through the abolishment of LPL-GPIHBP1 binding are . Because the LPL-GPIHBP1 complex is bound together primarily by hydrophobic interactions, switching a nonpolar methionine residue to a positively charged arginine residue, or a nonpolar cysteine residue to a polar tyrosine residue will disrupt these hydrophobic interactions and weaken the interface. Also, the much larger side chain of arginine is difficult to fit into the hydrophobic pocket of GPIHBP1. [6] These mutations do not affect LPL’s activity or secretion but play a role its ability to be transported to its site of action in the capillary lumen due to the inability of GPIHBP1 to bind and transport LPL. This leads to an accumulation of unmetabolized lipids but also catalytically active LPL in the interstitial spaces.[2]
Additionally, the carboxylic acid side chain of D201 is critical for coordinating LPL’s calcium ion, and the point mutations found in patients with chylomicronemia have been observed to eliminate LPL secretion and reduce its activity.[4] Mutating this negatively charged aspartic acid into a nonpolar valine residue disrupts the ionic bond between calcium and the aspartate, disturbing the overall calcium binding. A similar mutation has also been observed for D202E. These two mutations, in turn, destabilize LPL folding and thereby prevent its secretion from cells.[6] Knowledge about this enzyme's structure, function, and mutations will help create potential therapeutics to treat chylomicronemia and hypertriglyceridemia, reducing the risk of developing cardiovascular problems.[3]

![Figure 2. A cartoon representation of GPIHBP1's N-terminal intrinsically disordered region (IDR) colored green and an surface representation of LPL with electrostatic coloring [acidic (red), neutral (white), basic (blue)] to show how GPIHBP1's N-terminal domain interacts with LPL's basic patch to stabilize LPL structure and activity.](/wiki/images/thumb/7/72/Electro.jpg/250px-Electro.jpg)


