This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
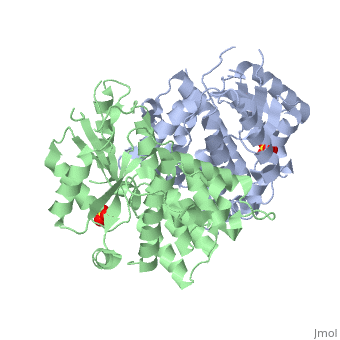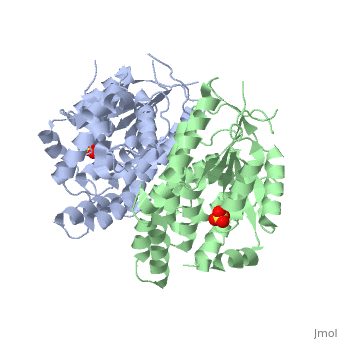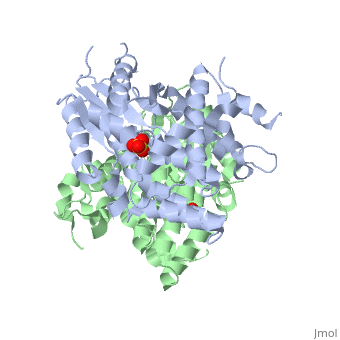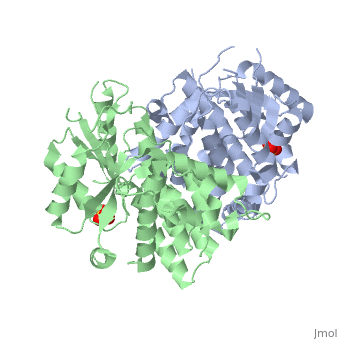
1e2h
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='1e2h' size='340' side='right'caption='[[1e2h]], [[Resolution|resolution]] 1.90Å' scene=''> | <StructureSection load='1e2h' size='340' side='right'caption='[[1e2h]], [[Resolution|resolution]] 1.90Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1e2h]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/ | + | <table><tr><td colspan='2'>[[1e2h]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Human_alphaherpesvirus_1_strain_17 Human alphaherpesvirus 1 strain 17]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E2H OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1E2H FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.9Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> |
| - | + | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1e2h FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e2h OCA], [https://pdbe.org/1e2h PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1e2h RCSB], [https://www.ebi.ac.uk/pdbsum/1e2h PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1e2h ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1e2h FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e2h OCA], [https://pdbe.org/1e2h PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1e2h RCSB], [https://www.ebi.ac.uk/pdbsum/1e2h PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1e2h ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/KITH_HHV11 KITH_HHV11] In latent infection, may allow the virus to be reactivated and to grow in cells lacking a high concentration of phosphorylated nucleic acid precursors, such as nerve cells that do not replicate their genome (By similarity). | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 32: | Line 31: | ||
==See Also== | ==See Also== | ||
| - | *[[Thymidine kinase|Thymidine kinase]] | + | *[[Thymidine kinase 3D structures|Thymidine kinase 3D structures]] |
== References == | == References == | ||
<references/> | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Human alphaherpesvirus 1 strain 17]] |
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | + | [[Category: Scapozza L]] | |
| - | [[Category: Scapozza | + | [[Category: Schulz GE]] |
| - | [[Category: Schulz | + | [[Category: Vogt J]] |
| - | [[Category: Vogt | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
The nucleoside binding site of Herpes simplex type 1 thymidine kinase analyzed by X-ray crystallography
| |||||||||||