We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1d5r
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
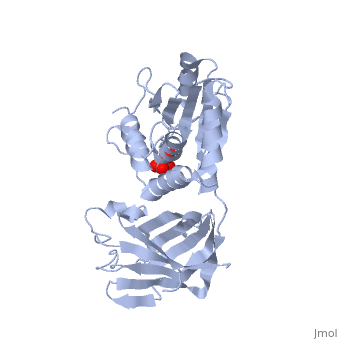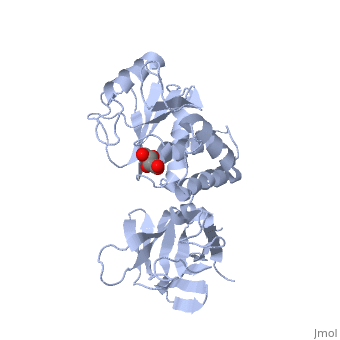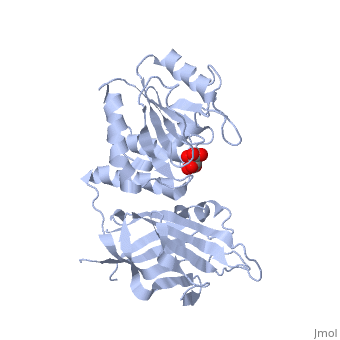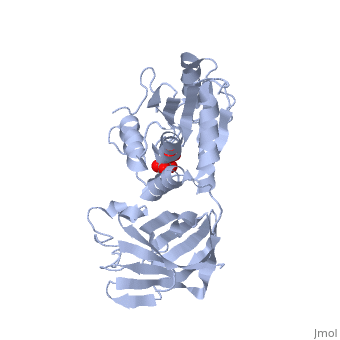
<StructureSection load='1d5r' size='340' side='right'caption='[[1d5r]], [[Resolution|resolution]] 2.10Å' scene=''> | <StructureSection load='1d5r' size='340' side='right'caption='[[1d5r]], [[Resolution|resolution]] 2.10Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1d5r]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/ | + | <table><tr><td colspan='2'>[[1d5r]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1D5R OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1D5R FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.1Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=TLA:L(+)-TARTARIC+ACID'>TLA</scene></td></tr> |
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1d5r FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1d5r OCA], [https://pdbe.org/1d5r PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1d5r RCSB], [https://www.ebi.ac.uk/pdbsum/1d5r PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1d5r ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1d5r FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1d5r OCA], [https://pdbe.org/1d5r PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1d5r RCSB], [https://www.ebi.ac.uk/pdbsum/1d5r PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1d5r ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Disease == | == Disease == | ||
| - | + | [https://www.uniprot.org/uniprot/PTEN_HUMAN PTEN_HUMAN] Defects in PTEN are a cause of Cowden disease (CD) [MIM:[https://omim.org/entry/158350 158350]; also known as Cowden syndrome (CS). CD is an autosomal dominant cancer predisposition syndrome associated with elevated risk for tumors of the breast, thyroid and skin. The predominant phenotype for CD is multiple hamartoma syndrome, in many organ systems including the breast (70% of CD patients), thyroid (40-60%), skin, CNS (40%), gastrointestinal tract. Affected individuals are at an increased risk of both breast and thyroid cancers. Trichilemmomas (benign tumors of the hair follicle infundibulum), and mucocutaneous papillomatosis (99%) are hallmarks of CD.<ref>PMID:9256433</ref> <ref>PMID:9811831</ref> <ref>PMID:9345101</ref> <ref>PMID:9399897</ref> <ref>PMID:9259288</ref> <ref>PMID:9140396</ref> <ref>PMID:9797362</ref> <ref>PMID:9600246</ref> <ref>PMID:9467011</ref> <ref>PMID:9735393</ref> <ref>PMID:9832031</ref> <ref>PMID:9425889</ref> <ref>PMID:9915974</ref> <ref>PMID:10051160</ref> <ref>PMID:10234502</ref> <ref>PMID:10866302</ref> <ref>PMID:11230179</ref> <ref>PMID:11494117</ref> Defects in PTEN are the cause of Lhermitte-Duclos disease (LDD) [MIM:[https://omim.org/entry/158350 158350]; also known as cerebelloparenchymal disorder VI. LDD is characterized by dysplastic gangliocytoma of the cerebellum which often results in cerebellar signs and seizures. LDD and CD seem to be the same entity, and are considered as hamartoma-neoplasia syndromes. Defects in PTEN are a cause of Bannayan-Zonana syndrome (BZS) [MIM:[https://omim.org/entry/153480 153480]; also known as Ruvalcaba-Myhre-Smith syndrome (RMSS) or Bannayan-Riley-Ruvalcaba syndrome (BRRS). In BZS there seems not to be an increased risk of malignancy. It has a partial clinical overlap with CD. BZS is characterized by the classic triad of macrocephaly, lipomatosis and pigmented macules of the gland penis.<ref>PMID:9467011</ref> <ref>PMID:10866302</ref> <ref>PMID:11494117</ref> <ref>PMID:9241266</ref> <ref>PMID:10400993</ref> Defects in PTEN are a cause of head and neck squamous cell carcinomas (HNSCC) [MIM:[https://omim.org/entry/275355 275355]; also known as squamous cell carcinoma of the head and neck.<ref>PMID:11801303</ref> Defects in PTEN are a cause of susceptibility to endometrial cancer (ENDMC) [MIM:[https://omim.org/entry/608089 608089]. Note=PTEN mutations are found in a subset of patients with Proteus syndrome, a genetically heterogeneous condition. The molecular diagnosis of PTEN mutation positive cases classifies Proteus syndrome patients as part of the PTEN hamartoma syndrome spectrum. As such, patients surviving the early years of Proteus syndrome are likely at a greater risk of developing malignancies. Defects in PTEN are a cause of susceptibility to glioma type 2 (GLM2) [MIM:[https://omim.org/entry/613028 613028]. Gliomas are central nervous system neoplasms derived from glial cells and comprise astrocytomas, glioblastoma multiforme, oligodendrogliomas, and ependymomas. Defects in PTEN are a cause of VACTERL association with hydrocephalus (VACTERL-H) [MIM:[https://omim.org/entry/276950 276950]. VACTERL is an acronym for vertebral anomalies, anal atresia, congenital cardiac disease, tracheoesophageal fistula, renal anomalies, radial dysplasia, and other limb defects. Defects in PTEN may be a cause of susceptibility to prostate cancer (PC) [MIM:[https://omim.org/entry/176807 176807]. It is a malignancy originating in tissues of the prostate. Most prostate cancers are adenocarcinomas that develop in the acini of the prostatic ducts. Other rare histopathologic types of prostate cancer that occur in approximately 5% of patients include small cell carcinoma, mucinous carcinoma, prostatic ductal carcinoma, transitional cell carcinoma, squamous cell carcinoma, basal cell carcinoma, adenoid cystic carcinoma (basaloid), signet-ring cell carcinoma and neuroendocrine carcinoma. Defects in PTEN are a cause of macrocephaly/autism syndrome (MCEPHAS) [MIM:[https://omim.org/entry/605309 605309]. Patients have autism spectrum disorders and macrocephaly, with head circumferences ranging from +2.5 to +8 SD for age and sex (average head circumference +4.0 SD).<ref>PMID:15805158</ref> Note=A microdeletion of chromosome 10q23 involving BMPR1A and PTEN is a cause of chromosome 10q23 deletion syndrome, which shows overlapping features of the following three disorders: Bannayan-Zonana syndrome, Cowden disease and juvenile polyposis syndrome. | |
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/PTEN_HUMAN PTEN_HUMAN] Tumor suppressor. Acts as a dual-specificity protein phosphatase, dephosphorylating tyrosine-, serine- and threonine-phosphorylated proteins. Also acts as a lipid phosphatase, removing the phosphate in the D3 position of the inositol ring from phosphatidylinositol 3,4,5-trisphosphate, phosphatidylinositol 3,4-diphosphate, phosphatidylinositol 3-phosphate and inositol 1,3,4,5-tetrakisphosphate with order of substrate preference in vitro PtdIns(3,4,5)P3 > PtdIns(3,4)P2 > PtdIns3P > Ins(1,3,4,5)P4. The lipid phosphatase activity is critical for its tumor suppressor function. Antagonizes the PI3K-AKT/PKB signaling pathway by dephosphorylating phosphoinositides and thereby modulating cell cycle progression and cell survival. The unphosphorylated form cooperates with AIP1 to suppress AKT1 activation. Dephosphorylates tyrosine-phosphorylated focal adhesion kinase and inhibits cell migration and integrin-mediated cell spreading and focal adhesion formation. Plays a role as a key modulator of the AKT-mTOR signaling pathway controlling the tempo of the process of newborn neurons integration during adult neurogenesis, including correct neuron positioning, dendritic development and synapse formation. May be a negative regulator of insulin signaling and glucose metabolism in adipose tissue. The nuclear monoubiquitinated form possesses greater apoptotic potential, whereas the cytoplasmic nonubiquitinated form induces less tumor suppressive ability.<ref>PMID:9187108</ref> <ref>PMID:9256433</ref> <ref>PMID:9593664</ref> <ref>PMID:9811831</ref> <ref>PMID:9616126</ref> <ref>PMID:10468583</ref> <ref>PMID:11707428</ref> <ref>PMID:18716620</ref> | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 22: | Line 22: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1d5r ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1d5r ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | The PTEN tumor suppressor is mutated in diverse human cancers and in hereditary cancer predisposition syndromes. PTEN is a phosphatase that can act on both polypeptide and phosphoinositide substrates in vitro. The PTEN structure reveals a phosphatase domain that is similar to protein phosphatases but has an enlarged active site important for the accommodation of the phosphoinositide substrate. The structure also reveals that PTEN has a C2 domain. The PTEN C2 domain binds phospholipid membranes in vitro, and mutation of basic residues that could mediate this reduces PTEN's membrane affinity and its ability to suppress the growth of glioblastoma tumor cells. The phosphatase and C2 domains associate across an extensive interface, suggesting that the C2 domain may serve to productively position the catalytic domain on the membrane. | ||
| - | |||
| - | Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association.,Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP Cell. 1999 Oct 29;99(3):323-34. PMID:10555148<ref>PMID:10555148</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1d5r" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
| Line 39: | Line 30: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Homo sapiens]] |
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: | + | [[Category: Di Cristofano A]] |
| - | + | [[Category: Georgescu M-M]] | |
| - | [[Category: Georgescu | + | [[Category: Lee JO]] |
| - | [[Category: Lee | + | [[Category: Pavletich NP]] |
| - | [[Category: Pavletich | + | [[Category: Yang H]] |
| - | [[Category: Yang | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
Crystal Structure of the PTEN Tumor Suppressor
| |||||||||||