We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
SN1 reaction
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
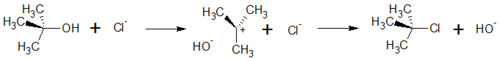
== S<sub>N</sub>1-Substitution of Cl<sup>-</sup> and ''tert''-Butanol == | == S<sub>N</sub>1-Substitution of Cl<sup>-</sup> and ''tert''-Butanol == | ||
| - | <StructureSection load='' size='340' side='right' caption='' scene='54/542276/Side_view/ | + | <StructureSection load='' size='340' side='right' caption='' scene='54/542276/Side_view/3'> |
The S<sub>N</sub>1 reaction belongs to the basic reaction in organic chemistry. The number 1 says that it is a monomolecular reaction. This means that in the rate determining step of the reaction, only one of the educts is involved. The kinetic of the reaction therefore follows the reation rate of first order. | The S<sub>N</sub>1 reaction belongs to the basic reaction in organic chemistry. The number 1 says that it is a monomolecular reaction. This means that in the rate determining step of the reaction, only one of the educts is involved. The kinetic of the reaction therefore follows the reation rate of first order. | ||
Revision as of 16:27, 8 July 2021
SN1-Substitution of Cl- and tert-Butanol
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Jaime Prilusky, Angel Herraez, Verena Pietzner