|
|
| Line 3: |
Line 3: |
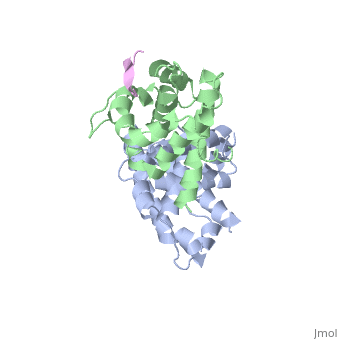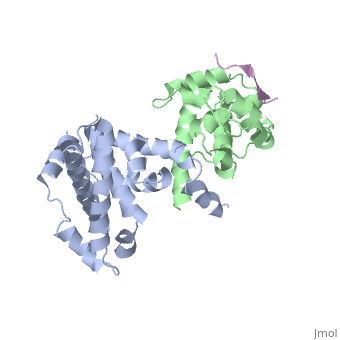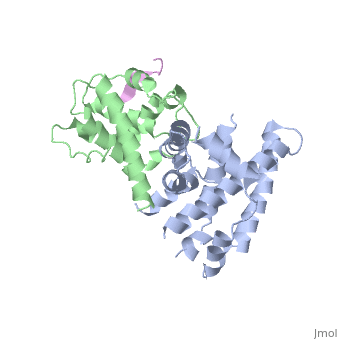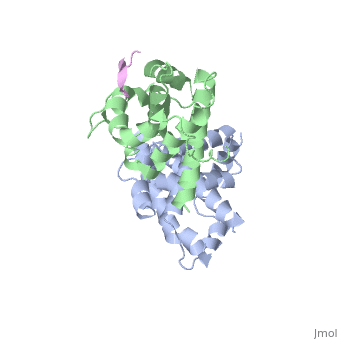
| | <StructureSection load='1gux' size='340' side='right'caption='[[1gux]], [[Resolution|resolution]] 1.85Å' scene=''> | | <StructureSection load='1gux' size='340' side='right'caption='[[1gux]], [[Resolution|resolution]] 1.85Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[1gux]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Human Human] and [https://en.wikipedia.org/wiki/Human_papillomavirus Human papillomavirus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1GUX OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1GUX FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[1gux]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [https://en.wikipedia.org/wiki/Human_papillomavirus Human papillomavirus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1GUX OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1GUX FirstGlance]. <br> |
| - | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1gux FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1gux OCA], [https://pdbe.org/1gux PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1gux RCSB], [https://www.ebi.ac.uk/pdbsum/1gux PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1gux ProSAT]</span></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.85Å</td></tr> |
| | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1gux FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1gux OCA], [https://pdbe.org/1gux PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1gux RCSB], [https://www.ebi.ac.uk/pdbsum/1gux PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1gux ProSAT]</span></td></tr> |
| | </table> | | </table> |
| | == Disease == | | == Disease == |
| - | [[https://www.uniprot.org/uniprot/RB_HUMAN RB_HUMAN]] Defects in RB1 are the cause of childhood cancer retinoblastoma (RB) [MIM:[https://omim.org/entry/180200 180200]]. RB is a congenital malignant tumor that arises from the nuclear layers of the retina. It occurs in about 1:20'000 live births and represents about 2% of childhood malignancies. It is bilateral in about 30% of cases. Although most RB appear sporadically, about 20% are transmitted as an autosomal dominant trait with incomplete penetrance. The diagnosis is usually made before the age of 2 years when strabismus or a gray to yellow reflex from pupil ('cat eye') is investigated.<ref>PMID:2594029</ref> <ref>PMID:1352883</ref> <ref>PMID:8346255</ref> <ref>PMID:7704558</ref> <ref>PMID:7927327</ref> <ref>PMID:8605116</ref> <ref>PMID:7795591</ref> <ref>PMID:8776589</ref> <ref>PMID:9311732</ref> <ref>PMID:9140452</ref> <ref>PMID:10671068</ref> <ref>PMID:9973307</ref> <ref>PMID:11524739</ref> Defects in RB1 are a cause of susceptibility to bladder cancer (BLC) [MIM:[https://omim.org/entry/109800 109800]]. A malignancy originating in tissues of the urinary bladder. It often presents with multiple tumors appearing at different times and at different sites in the bladder. Most bladder cancers are transitional cell carcinomas. They begin in cells that normally make up the inner lining of the bladder. Other types of bladder cancer include squamous cell carcinoma (cancer that begins in thin, flat cells) and adenocarcinoma (cancer that begins in cells that make and release mucus and other fluids). Bladder cancer is a complex disorder with both genetic and environmental influences. Defects in RB1 are a cause of osteogenic sarcoma (OSRC) [MIM:[https://omim.org/entry/259500 259500]].
| + | [https://www.uniprot.org/uniprot/RB_HUMAN RB_HUMAN] Defects in RB1 are the cause of childhood cancer retinoblastoma (RB) [MIM:[https://omim.org/entry/180200 180200]. RB is a congenital malignant tumor that arises from the nuclear layers of the retina. It occurs in about 1:20'000 live births and represents about 2% of childhood malignancies. It is bilateral in about 30% of cases. Although most RB appear sporadically, about 20% are transmitted as an autosomal dominant trait with incomplete penetrance. The diagnosis is usually made before the age of 2 years when strabismus or a gray to yellow reflex from pupil ('cat eye') is investigated.<ref>PMID:2594029</ref> <ref>PMID:1352883</ref> <ref>PMID:8346255</ref> <ref>PMID:7704558</ref> <ref>PMID:7927327</ref> <ref>PMID:8605116</ref> <ref>PMID:7795591</ref> <ref>PMID:8776589</ref> <ref>PMID:9311732</ref> <ref>PMID:9140452</ref> <ref>PMID:10671068</ref> <ref>PMID:9973307</ref> <ref>PMID:11524739</ref> Defects in RB1 are a cause of susceptibility to bladder cancer (BLC) [MIM:[https://omim.org/entry/109800 109800]. A malignancy originating in tissues of the urinary bladder. It often presents with multiple tumors appearing at different times and at different sites in the bladder. Most bladder cancers are transitional cell carcinomas. They begin in cells that normally make up the inner lining of the bladder. Other types of bladder cancer include squamous cell carcinoma (cancer that begins in thin, flat cells) and adenocarcinoma (cancer that begins in cells that make and release mucus and other fluids). Bladder cancer is a complex disorder with both genetic and environmental influences. Defects in RB1 are a cause of osteogenic sarcoma (OSRC) [MIM:[https://omim.org/entry/259500 259500]. |
| | == Function == | | == Function == |
| - | [[https://www.uniprot.org/uniprot/RB_HUMAN RB_HUMAN]] Key regulator of entry into cell division that acts as a tumor suppressor. Promotes G0-G1 transition when phosphorylated by CDK3/cyclin-C. Acts as a transcription repressor of E2F1 target genes. The underphosphorylated, active form of RB1 interacts with E2F1 and represses its transcription activity, leading to cell cycle arrest. Directly involved in heterochromatin formation by maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin by stabilizing histone methylation. Recruits and targets histone methyltransferases SUV39H1, SUV420H1 and SUV420H2, leading to epigenetic transcriptional repression. Controls histone H4 'Lys-20' trimethylation. Inhibits the intrinsic kinase activity of TAF1. Mediates transcriptional repression by SMARCA4/BRG1 by recruiting a histone deacetylase (HDAC) complex to the c-FOS promoter. In resting neurons, transcription of the c-FOS promoter is inhibited by BRG1-dependent recruitment of a phospho-RB1-HDAC1 repressor complex. Upon calcium influx, RB1 is dephosphorylated by calcineurin, which leads to release of the repressor complex (By similarity). In case of viral infections, interactions with SV40 large T antigen, HPV E7 protein or adenovirus E1A protein induce the disassembly of RB1-E2F1 complex thereby disrupting RB1's activity.<ref>PMID:15084261</ref>
| + | [https://www.uniprot.org/uniprot/RB_HUMAN RB_HUMAN] Key regulator of entry into cell division that acts as a tumor suppressor. Promotes G0-G1 transition when phosphorylated by CDK3/cyclin-C. Acts as a transcription repressor of E2F1 target genes. The underphosphorylated, active form of RB1 interacts with E2F1 and represses its transcription activity, leading to cell cycle arrest. Directly involved in heterochromatin formation by maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin by stabilizing histone methylation. Recruits and targets histone methyltransferases SUV39H1, SUV420H1 and SUV420H2, leading to epigenetic transcriptional repression. Controls histone H4 'Lys-20' trimethylation. Inhibits the intrinsic kinase activity of TAF1. Mediates transcriptional repression by SMARCA4/BRG1 by recruiting a histone deacetylase (HDAC) complex to the c-FOS promoter. In resting neurons, transcription of the c-FOS promoter is inhibited by BRG1-dependent recruitment of a phospho-RB1-HDAC1 repressor complex. Upon calcium influx, RB1 is dephosphorylated by calcineurin, which leads to release of the repressor complex (By similarity). In case of viral infections, interactions with SV40 large T antigen, HPV E7 protein or adenovirus E1A protein induce the disassembly of RB1-E2F1 complex thereby disrupting RB1's activity.<ref>PMID:15084261</ref> |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 20: |
Line 21: |
| | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1gux ConSurf]. | | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1gux ConSurf]. |
| | <div style="clear:both"></div> | | <div style="clear:both"></div> |
| - | <div style="background-color:#fffaf0;"> | |
| - | == Publication Abstract from PubMed == | |
| - | The pocket domain of the retinoblastoma (Rb) tumour suppressor is central to Rb function, and is frequently inactivated by the binding of the human papilloma virus E7 oncoprotein in cervical cancer. The crystal structure of the Rb pocket bound to a nine-residue E7 peptide containing the LxCxE motif, shared by other Rb-binding viral and cellular proteins, shows that the LxCxE peptide binds a highly conserved groove on the B-box portion of the pocket; the A-box portion appears to be required for the stable folding of the B box. Also highly conserved is the extensive A-B interface, suggesting that it may be an additional protein-binding site. The A and B boxes each contain the cyclin-fold structural motif, with the LxCxE-binding site on the B-box cyclin fold being similar to a Cdk2-binding site of cyclin A and to a TBP-binding site of TFIIB. | |
| - | | |
| - | Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7.,Lee JO, Russo AA, Pavletich NP Nature. 1998 Feb 26;391(6670):859-65. PMID:9495340<ref>PMID:9495340</ref> | |
| - | | |
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | </div> | |
| - | <div class="pdbe-citations 1gux" style="background-color:#fffaf0;"></div> | |
| | | | |
| | ==See Also== | | ==See Also== |
| Line 36: |
Line 28: |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Human]] | + | [[Category: Homo sapiens]] |
| | [[Category: Human papillomavirus]] | | [[Category: Human papillomavirus]] |
| | [[Category: Large Structures]] | | [[Category: Large Structures]] |
| - | [[Category: Lee, J O]] | + | [[Category: Lee JO]] |
| - | [[Category: Pavletich, N P]] | + | [[Category: Pavletich NP]] |
| - | [[Category: Russo, A A]] | + | [[Category: Russo AA]] |
| - | [[Category: Retinoblastoma]]
| + | |
| - | [[Category: Tumor suppressor protein]]
| + | |
| Structural highlights
Disease
RB_HUMAN Defects in RB1 are the cause of childhood cancer retinoblastoma (RB) [MIM:180200. RB is a congenital malignant tumor that arises from the nuclear layers of the retina. It occurs in about 1:20'000 live births and represents about 2% of childhood malignancies. It is bilateral in about 30% of cases. Although most RB appear sporadically, about 20% are transmitted as an autosomal dominant trait with incomplete penetrance. The diagnosis is usually made before the age of 2 years when strabismus or a gray to yellow reflex from pupil ('cat eye') is investigated.[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] Defects in RB1 are a cause of susceptibility to bladder cancer (BLC) [MIM:109800. A malignancy originating in tissues of the urinary bladder. It often presents with multiple tumors appearing at different times and at different sites in the bladder. Most bladder cancers are transitional cell carcinomas. They begin in cells that normally make up the inner lining of the bladder. Other types of bladder cancer include squamous cell carcinoma (cancer that begins in thin, flat cells) and adenocarcinoma (cancer that begins in cells that make and release mucus and other fluids). Bladder cancer is a complex disorder with both genetic and environmental influences. Defects in RB1 are a cause of osteogenic sarcoma (OSRC) [MIM:259500.
Function
RB_HUMAN Key regulator of entry into cell division that acts as a tumor suppressor. Promotes G0-G1 transition when phosphorylated by CDK3/cyclin-C. Acts as a transcription repressor of E2F1 target genes. The underphosphorylated, active form of RB1 interacts with E2F1 and represses its transcription activity, leading to cell cycle arrest. Directly involved in heterochromatin formation by maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin by stabilizing histone methylation. Recruits and targets histone methyltransferases SUV39H1, SUV420H1 and SUV420H2, leading to epigenetic transcriptional repression. Controls histone H4 'Lys-20' trimethylation. Inhibits the intrinsic kinase activity of TAF1. Mediates transcriptional repression by SMARCA4/BRG1 by recruiting a histone deacetylase (HDAC) complex to the c-FOS promoter. In resting neurons, transcription of the c-FOS promoter is inhibited by BRG1-dependent recruitment of a phospho-RB1-HDAC1 repressor complex. Upon calcium influx, RB1 is dephosphorylated by calcineurin, which leads to release of the repressor complex (By similarity). In case of viral infections, interactions with SV40 large T antigen, HPV E7 protein or adenovirus E1A protein induce the disassembly of RB1-E2F1 complex thereby disrupting RB1's activity.[14]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
See Also
References
- ↑ Yandell DW, Campbell TA, Dayton SH, Petersen R, Walton D, Little JB, McConkie-Rosell A, Buckley EG, Dryja TP. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689-95. PMID:2594029
- ↑ Onadim Z, Hogg A, Baird PN, Cowell JK. Oncogenic point mutations in exon 20 of the RB1 gene in families showing incomplete penetrance and mild expression of the retinoblastoma phenotype. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6177-81. PMID:1352883
- ↑ Hogg A, Bia B, Onadim Z, Cowell JK. Molecular mechanisms of oncogenic mutations in tumors from patients with bilateral and unilateral retinoblastoma. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7351-5. PMID:8346255
- ↑ Cowell JK, Smith T, Bia B. Frequent constitutional C to T mutations in CGA-arginine codons in the RB1 gene produce premature stop codons in patients with bilateral (hereditary) retinoblastoma. Eur J Hum Genet. 1994;2(4):281-90. PMID:7704558
- ↑ Lohmann DR, Brandt B, Hopping W, Passarge E, Horsthemke B. Distinct RB1 gene mutations with low penetrance in hereditary retinoblastoma. Hum Genet. 1994 Oct;94(4):349-54. PMID:7927327
- ↑ Liu Z, Song Y, Bia B, Cowell JK. Germline mutations in the RB1 gene in patients with hereditary retinoblastoma. Genes Chromosomes Cancer. 1995 Dec;14(4):277-84. PMID:8605116
- ↑ Blanquet V, Turleau C, Gross-Morand MS, Senamaud-Beaufort C, Doz F, Besmond C. Spectrum of germline mutations in the RB1 gene: a study of 232 patients with hereditary and non hereditary retinoblastoma. Hum Mol Genet. 1995 Mar;4(3):383-8. PMID:7795591
- ↑ Van Orsouw NJ, Li D, van der Vlies P, Scheffer H, Eng C, Buys CH, Li FP, Vijg J. Mutational scanning of large genes by extensive PCR multiplexing and two-dimensional electrophoresis: application to the RB1 gene. Hum Mol Genet. 1996 Jun;5(6):755-61. PMID:8776589
- ↑ Lohmann DR, Gerick M, Brandt B, Oelschlager U, Lorenz B, Passarge E, Horsthemke B. Constitutional RB1-gene mutations in patients with isolated unilateral retinoblastoma. Am J Hum Genet. 1997 Aug;61(2):282-94. PMID:9311732 doi:10.1086/514845
- ↑ Mateu E, Sanchez F, Najera C, Beneyto M, Castell V, Hernandez M, Serra I, Prieto F. Genetics of retinoblastoma: a study. Cancer Genet Cytogenet. 1997 May;95(1):40-50. PMID:9140452
- ↑ Yilmaz S, Horsthemke B, Lohmann DR. Twelve novel RB1 gene mutations in patients with hereditary retinoblastoma. Mutations in brief no. 206. Online. Hum Mutat. 1998;12(6):434. PMID:10671068 doi:<434::AID-HUMU15>3.0.CO;2-A 10.1002/(SICI)1098-1004(1998)12:6<434::AID-HUMU15>3.0.CO;2-A
- ↑ Klutz M, Horsthemke B, Lohmann DR. RB1 gene mutations in peripheral blood DNA of patients with isolated unilateral retinoblastoma. Am J Hum Genet. 1999 Feb;64(2):667-8. PMID:9973307 doi:10.1086/302254
- ↑ Yu YS, Kim IJ, Ku JL, Park JG. Identification of four novel RB1 germline mutations in Korean retinoblastoma patients. Hum Mutat. 2001 Sep;18(3):252. PMID:11524739 doi:10.1002/humu.1184
- ↑ Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004 Apr 16;117(2):239-51. PMID:15084261
|