|
|
| Line 3: |
Line 3: |
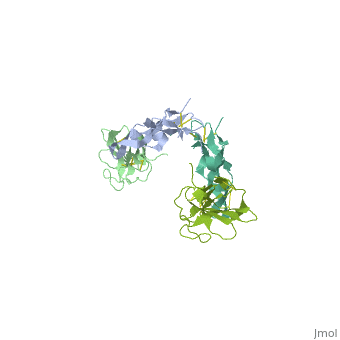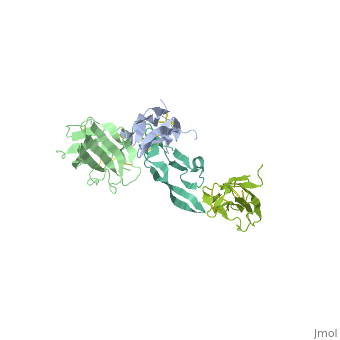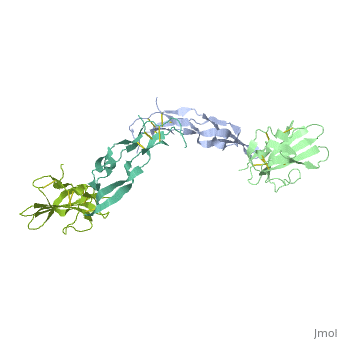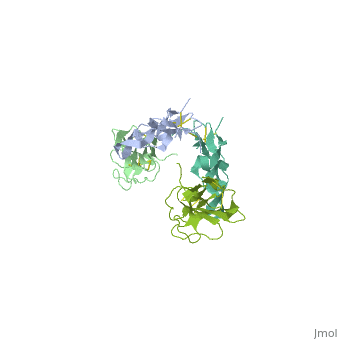
| | <StructureSection load='1ktz' size='340' side='right'caption='[[1ktz]], [[Resolution|resolution]] 2.15Å' scene=''> | | <StructureSection load='1ktz' size='340' side='right'caption='[[1ktz]], [[Resolution|resolution]] 2.15Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[1ktz]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1KTZ OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1KTZ FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[1ktz]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1KTZ OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1KTZ FirstGlance]. <br> |
| - | </td></tr><tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[https://en.wikipedia.org/wiki/Transferase Transferase], with EC number [https://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.11.1, 2.7.11.8, 2.7.11.9, 2.7.11.10, 2.7.11.11, 2.7.11.12, 2.7.11.13, 2.7.11.21, 2.7.11.22, 2.7.11.24, 2.7.11.25, 2.7.11.30 and 2.7.12.1 2.7.11.1, 2.7.11.8, 2.7.11.9, 2.7.11.10, 2.7.11.11, 2.7.11.12, 2.7.11.13, 2.7.11.21, 2.7.11.22, 2.7.11.24, 2.7.11.25, 2.7.11.30 and 2.7.12.1] </span></td></tr> | + | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1ktz FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ktz OCA], [https://pdbe.org/1ktz PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1ktz RCSB], [https://www.ebi.ac.uk/pdbsum/1ktz PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1ktz ProSAT]</span></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1ktz FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ktz OCA], [https://pdbe.org/1ktz PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1ktz RCSB], [https://www.ebi.ac.uk/pdbsum/1ktz PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1ktz ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| | == Disease == | | == Disease == |
| - | [[https://www.uniprot.org/uniprot/TGFB3_HUMAN TGFB3_HUMAN]] Defects in TGFB3 are a cause of familial arrhythmogenic right ventricular dysplasia type 1 (ARVD1) [MIM:[https://omim.org/entry/107970 107970]]; also known as arrhythmogenic right ventricular cardiomyopathy 1 (ARVC1). ARVD is an autosomal dominant disease characterized by partial degeneration of the myocardium of the right ventricle, electrical instability, and sudden death. It is clinically defined by electrocardiographic and angiographic criteria; pathologic findings, replacement of ventricular myocardium with fatty and fibrous elements, preferentially involve the right ventricular free wall.<ref>PMID:15639475</ref> [[https://www.uniprot.org/uniprot/TGFR2_HUMAN TGFR2_HUMAN]] Defects in TGFBR2 are the cause of hereditary non-polyposis colorectal cancer type 6 (HNPCC6) [MIM:[https://omim.org/entry/614331 614331]]. Mutations in more than one gene locus can be involved alone or in combination in the production of the HNPCC phenotype (also called Lynch syndrome). Most families with clinically recognized HNPCC have mutations in either MLH1 or MSH2 genes. HNPCC is an autosomal, dominantly inherited disease associated with marked increase in cancer susceptibility. It is characterized by a familial predisposition to early onset colorectal carcinoma (CRC) and extra-colonic cancers of the gastrointestinal, urological and female reproductive tracts. HNPCC is reported to be the most common form of inherited colorectal cancer in the Western world, and accounts for 15% of all colon cancers. Cancers in HNPCC originate within benign neoplastic polyps termed adenomas. Clinically, HNPCC is often divided into two subgroups. Type I: hereditary predisposition to colorectal cancer, a young age of onset, and carcinoma observed in the proximal colon. Type II: patients have an increased risk for cancers in certain tissues such as the uterus, ovary, breast, stomach, small intestine, skin, and larynx in addition to the colon. Diagnosis of classical HNPCC is based on the Amsterdam criteria: 3 or more relatives affected by colorectal cancer, one a first degree relative of the other two; 2 or more generation affected; 1 or more colorectal cancers presenting before 50 years of age; exclusion of hereditary polyposis syndromes. The term "suspected HNPCC" or "incomplete HNPCC" can be used to describe families who do not or only partially fulfill the Amsterdam criteria, but in whom a genetic basis for colon cancer is strongly suspected. HNPCC6 is a type of colorectal cancer complying with the clinical criteria of HNPCC, except that the onset of cancer was beyond 50 years of age in all cases.<ref>PMID:9590282</ref> Defects in TGFBR2 are a cause of esophageal cancer (ESCR) [MIM:[https://omim.org/entry/133239 133239]]. Defects in TGFBR2 are the cause of Loeys-Dietz syndrome type 1B (LDS1B) [MIM:[https://omim.org/entry/610168 610168]]. LDS1 is an aortic aneurysm syndrome with widespread systemic involvement. The disorder is characterized by arterial tortuosity and aneurysms, craniosynostosis, hypertelorism, and bifid uvula or cleft palate. Other findings include exotropy, micrognathia and retrognathia, structural brain abnormalities, intellectual deficit, congenital heart disease, translucent skin, joint hyperlaxity and aneurysm with dissection throughout the arterial tree.<ref>PMID:15731757</ref> <ref>PMID:16251899</ref> <ref>PMID:20101701</ref> <ref>PMID:20358619</ref> <ref>PMID:22113417</ref> <ref>PMID:21949523</ref> Defects in TGFBR2 are the cause of Loeys-Dietz syndrome type 2B (LDS2B) [MIM:[https://omim.org/entry/610380 610380]]. An aortic aneurysm syndrome with widespread systemic involvement. Physical findings include prominent joint laxity, easy bruising, wide and atrophic scars, velvety and translucent skin with easily visible veins, spontaneous rupture of the spleen or bowel, diffuse arterial aneurysms and dissections, and catastrophic complications of pregnancy, including rupture of the gravid uterus and the arteries, either during pregnancy or in the immediate postpartum period. LDS2 is characterized by the absence of craniofacial abnormalities with the exception of bifid uvula that can be present in some patients. Note=TGFBR2 mutations Cys-460 and His-460 have been reported to be associated with thoracic aortic aneurysms and dissection (TAAD). This phenotype, also known as thoracic aortic aneurysms type 3 (AAT3), is distinguised from LDS2B by having aneurysms restricted to thoracic aorta. As individuals carrying these mutations also exhibit descending aortic disease and aneurysms of other arteries (PubMed:16027248), they have been considered as LDS2B by the OMIM resource.
| + | [https://www.uniprot.org/uniprot/TGFB3_HUMAN TGFB3_HUMAN] Defects in TGFB3 are a cause of familial arrhythmogenic right ventricular dysplasia type 1 (ARVD1) [MIM:[https://omim.org/entry/107970 107970]; also known as arrhythmogenic right ventricular cardiomyopathy 1 (ARVC1). ARVD is an autosomal dominant disease characterized by partial degeneration of the myocardium of the right ventricle, electrical instability, and sudden death. It is clinically defined by electrocardiographic and angiographic criteria; pathologic findings, replacement of ventricular myocardium with fatty and fibrous elements, preferentially involve the right ventricular free wall.<ref>PMID:15639475</ref> |
| | == Function == | | == Function == |
| - | [[https://www.uniprot.org/uniprot/TGFB3_HUMAN TGFB3_HUMAN]] Involved in embryogenesis and cell differentiation. [[https://www.uniprot.org/uniprot/TGFR2_HUMAN TGFR2_HUMAN]] Transmembrane serine/threonine kinase forming with the TGF-beta type I serine/threonine kinase receptor, TGFBR1, the non-promiscuous receptor for the TGF-beta cytokines TGFB1, TGFB2 and TGFB3. Transduces the TGFB1, TGFB2 and TGFB3 signal from the cell surface to the cytoplasm and is thus regulating a plethora of physiological and pathological processes including cell cycle arrest in epithelial and hematopoietic cells, control of mesenchymal cell proliferation and differentiation, wound healing, extracellular matrix production, immunosuppression and carcinogenesis. The formation of the receptor complex composed of 2 TGFBR1 and 2 TGFBR2 molecules symmetrically bound to the cytokine dimer results in the phosphorylation and the activation of TGFRB1 by the constitutively active TGFBR2. Activated TGFBR1 phosphorylates SMAD2 which dissociates from the receptor and interacts with SMAD4. The SMAD2-SMAD4 complex is subsequently translocated to the nucleus where it modulates the transcription of the TGF-beta-regulated genes. This constitutes the canonical SMAD-dependent TGF-beta signaling cascade. Also involved in non-canonical, SMAD-independent TGF-beta signaling pathways.<ref>PMID:7774578</ref>
| + | [https://www.uniprot.org/uniprot/TGFB3_HUMAN TGFB3_HUMAN] Involved in embryogenesis and cell differentiation. |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 33: |
Line 32: |
| | ==See Also== | | ==See Also== |
| | *[[TGF-beta receptor|TGF-beta receptor]] | | *[[TGF-beta receptor|TGF-beta receptor]] |
| - | *[[TGF-ò receptor 3D structures|TGF-ò receptor 3D structures]] | + | *[[TGF-beta receptor 3D structures|TGF-beta receptor 3D structures]] |
| | == References == | | == References == |
| | <references/> | | <references/> |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Human]] | + | [[Category: Homo sapiens]] |
| | [[Category: Large Structures]] | | [[Category: Large Structures]] |
| - | [[Category: Transferase]]
| + | [[Category: Deep S]] |
| - | [[Category: Deep, S]] | + | [[Category: Hart PJ]] |
| - | [[Category: Hart, P J]] | + | [[Category: Hinck AP]] |
| - | [[Category: Hinck, A P]] | + | [[Category: Hinck CS]] |
| - | [[Category: Hinck, C S]] | + | [[Category: Shu Z]] |
| - | [[Category: Shu, Z]] | + | [[Category: Taylor AB]] |
| - | [[Category: Taylor, A B]] | + | |
| - | [[Category: Cytokine-cytokine receptor complex]]
| + | |
| - | [[Category: Cytokine-receptor complex]]
| + | |
| Structural highlights
Disease
TGFB3_HUMAN Defects in TGFB3 are a cause of familial arrhythmogenic right ventricular dysplasia type 1 (ARVD1) [MIM:107970; also known as arrhythmogenic right ventricular cardiomyopathy 1 (ARVC1). ARVD is an autosomal dominant disease characterized by partial degeneration of the myocardium of the right ventricle, electrical instability, and sudden death. It is clinically defined by electrocardiographic and angiographic criteria; pathologic findings, replacement of ventricular myocardium with fatty and fibrous elements, preferentially involve the right ventricular free wall.[1]
Function
TGFB3_HUMAN Involved in embryogenesis and cell differentiation.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Transforming growth factor-beta (TGF-beta) is the prototype of a large family of structurally related cytokines that play key roles in maintaining cellular homeostasis by signaling through two classes of functionally distinct Ser/Thr kinase receptors, designated as type I and type II. TGF-beta initiates receptor assembly by binding with high affinity to the type II receptor. Here, we present the 2.15 A crystal structure of the extracellular ligand-binding domain of the human TGF-beta type II receptor (ecTbetaR2) in complex with human TGF-beta3. ecTbetaR2 interacts with homodimeric TGF-beta3 by binding identical finger segments at opposite ends of the growth factor. Relative to the canonical 'closed' conformation previously observed in ligand structures across the superfamily, ecTbetaR2-bound TGF-beta3 shows an altered arrangement of its monomeric subunits, designated the 'open' conformation. The mode of TGF-beta3 binding shown by ecTbetaR2 is compatible with both ligand conformations. This, in addition to the predicted mode for TGF-beta binding to the type I receptor ectodomain (ecTbetaR1), suggests an assembly mechanism in which ecTbetaR1 and ecTbetaR2 bind at adjacent positions on the ligand surface and directly contact each other via protein--protein interactions.
Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex.,Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP Nat Struct Biol. 2002 Mar;9(3):203-8. PMID:11850637[2]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Beffagna G, Occhi G, Nava A, Vitiello L, Ditadi A, Basso C, Bauce B, Carraro G, Thiene G, Towbin JA, Danieli GA, Rampazzo A. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res. 2005 Feb 1;65(2):366-73. PMID:15639475 doi:S0008-6363(04)00440-7
- ↑ Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex. Nat Struct Biol. 2002 Mar;9(3):203-8. PMID:11850637 doi:http://dx.doi.org/10.1038/nsb766
|