Receptor protein serine/threonine kinases
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
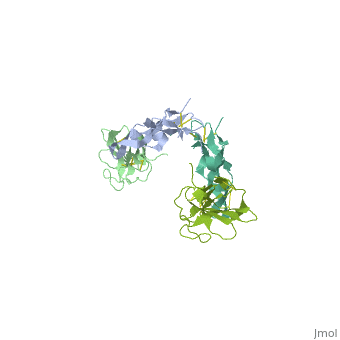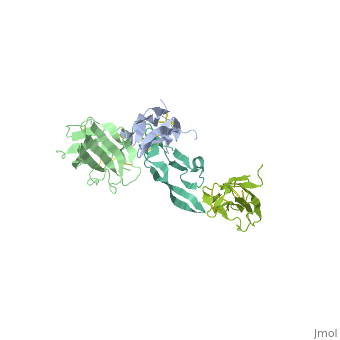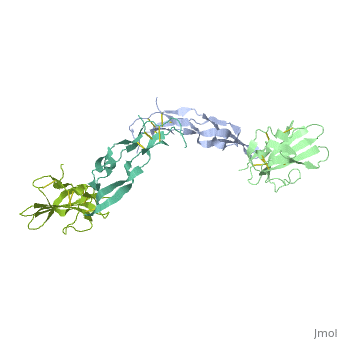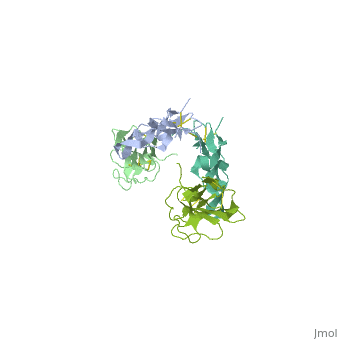
<StructureSection load='1ktz' size='340' side='right' caption='Human hTGFR-II extracellular domain (green) complex with TGF-β3 (grey) (PDB code [[1ktz]])' scene=''> | <StructureSection load='1ktz' size='340' side='right' caption='Human hTGFR-II extracellular domain (green) complex with TGF-β3 (grey) (PDB code [[1ktz]])' scene=''> | ||
See also [[Serine/threonine protein kinase]]. | See also [[Serine/threonine protein kinase]]. | ||
| + | |||
| + | The systematic name of this enzyme class is ATP:receptor-protein phosphotransferase. Proteins from this group participate in 7 metabolic pathways: MAPK signaling pathway, cytokine-cytokine receptor interaction, TGF beta signaling pathway, adherens junction, colorectal cancer, pancreatic cancer, and chronic myeloid leukemia. | ||
| + | |||
==TGF-beta receptor== | ==TGF-beta receptor== | ||
*[[TGF-beta receptor]] | *[[TGF-beta receptor]] | ||
Current revision
| |||||||||||
References
- ↑ Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem. 2014 Oct 9;57(19):7900-15. doi: 10.1021/jm501177w. Epub 2014 Sep 4. PMID:25101911 doi:http://dx.doi.org/10.1021/jm501177w
- ↑ Chen H, Kong Y, Chen J, Li L, Li X, Yu F, Ming Z. Crystal structure of the extracellular domain of the receptor-like kinase TMK3 from Arabidopsis thaliana. Acta Crystallogr F Struct Biol Commun. 2020 Aug 1;76(Pt 8):384-390. doi:, 10.1107/S2053230X20010122. Epub 2020 Jul 29. PMID:32744250 doi:http://dx.doi.org/10.1107/S2053230X20010122