Journal:Acta Cryst F:S2053230X21008967
From Proteopedia
(Difference between revisions)

| Line 8: | Line 8: | ||
The overall structure of AGAO determined by SFX had almost the same conformation as those determined previously by synchrotron X-ray and neutron crystallography performed at cryogenic temperature. The electron-density map in the active site clearly showed the resting form of the protein-derived cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ). In addition, the active-site Cu2+ was ligated with three histidine residues and two water molecules that are located at positions identical to those determined by the previous studies. These results show that the bound Cu2+ in AGAO is resistant to X-ray photoreduction, which is accompanied by conformational changes of the metal coordination structure. The availability of high-quality microcrystals in large quantities is promising for studying the structural changes of AGAO during the catalytic reaction by the ‘mix-and-inject’ time-resolved SFX. | The overall structure of AGAO determined by SFX had almost the same conformation as those determined previously by synchrotron X-ray and neutron crystallography performed at cryogenic temperature. The electron-density map in the active site clearly showed the resting form of the protein-derived cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ). In addition, the active-site Cu2+ was ligated with three histidine residues and two water molecules that are located at positions identical to those determined by the previous studies. These results show that the bound Cu2+ in AGAO is resistant to X-ray photoreduction, which is accompanied by conformational changes of the metal coordination structure. The availability of high-quality microcrystals in large quantities is promising for studying the structural changes of AGAO during the catalytic reaction by the ‘mix-and-inject’ time-resolved SFX. | ||
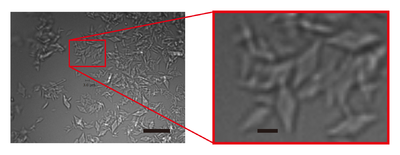
| - | [[Image:Image_1_0a.png|left|400px|thumb|'''Figure 1'' Images of AGAO microcrystals grown by combining micro-seeding and batch | + | [[Image:Image_1_0a.png|left|400px|thumb|'''Figure 1''' Images of AGAO microcrystals grown by combining micro-seeding and batch |
crystallisation. The right panel shows an enlarged view of the part indicated by a red rectangle in the | crystallisation. The right panel shows an enlarged view of the part indicated by a red rectangle in the | ||
left panel. Scale bars in the left and right panels represent 20 and 3 µm, respectively.]] | left panel. Scale bars in the left and right panels represent 20 and 3 µm, respectively.]] | ||
Revision as of 13:30, 9 September 2021
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.