Porin
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
*[[Molecular Playground/OmpG2]]<br /> | *[[Molecular Playground/OmpG2]]<br /> | ||
*[[Osmoporin OmpK36 (K. pneumoniae)]]<br /> | *[[Osmoporin OmpK36 (K. pneumoniae)]]<br /> | ||
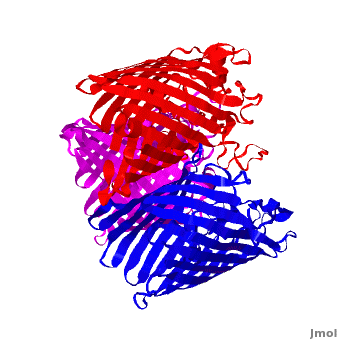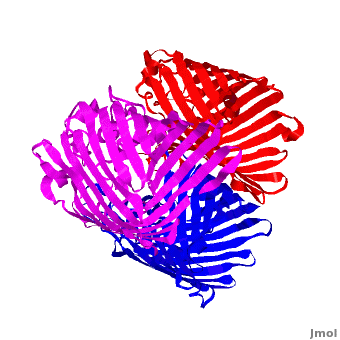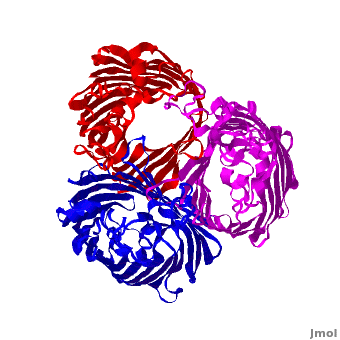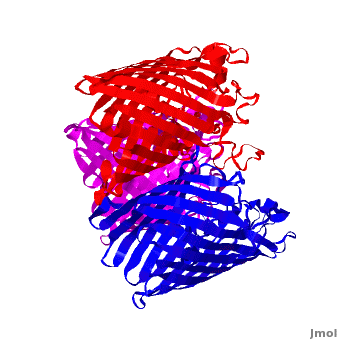
| - | '''Voltage-Dependent Anion Channel (VDAC)''' is ion channel Omp found in outer mitochondrial membrane. In Pseudomonas aeruginosa the porin gene products are named OprD, OprK, OprP. The images at the left and at the right correspond to one representative porin structure, ''i.e.'' crystal structure osmoporin OmpC from ''Escherichia coli'' ([[2j1n]]). OmpC has three beta-barrels associated to form a <scene name='Porin/Cv/2'>tight trimer</scene> <ref>PMID:16949612</ref>. Porin is a transmembrane protein, as can be | + | '''Voltage-Dependent Anion Channel (VDAC)''' is ion channel Omp found in outer mitochondrial membrane. In Pseudomonas aeruginosa the porin gene products are named OprD, OprK, OprP. The images at the left and at the right correspond to one representative porin structure, ''i.e.'' crystal structure osmoporin OmpC from ''Escherichia coli'' ([[2j1n]]). OmpC has three beta-barrels associated to form a <scene name='Porin/Cv/2'>tight trimer</scene> <ref>PMID:16949612</ref>. Porin is a transmembrane protein, as can be seen from the <jmol><jmolLink><script>script "/scripts/1a0s/Hidrophobic/1.spt"; ppdiaCaptionCmd = "changeCaption('Hydrophobic residues (shown in pink) are prevalent where the protein comes in contact with the hydrophbic layer of the double membrane. Shown here is the sucrose-specific porin (PDB-ID 1a0s) in its trimeric quaternary structure.','white','black');";javascript @ppdiaCaptionCmd;</script><text>hydrophobic ring</text></jmolLink></jmol> around the protein, this makes it possible to submerge in the lipid bilayer (hydrophobic amino acids are sandybrown, hydrophilic ones are cyan). As you can <scene name='1a0s/Hidrophobic1/1'>see</scene> the hole in the protein is made of mainly hydrophilic chains thus making it possible for the sugar to pass through (these scenes were created by Nádori Gergely). |
| - | < | + | |
== 3D structures of Porin == | == 3D structures of Porin == | ||
Revision as of 15:56, 5 August 2023
| |||||||||||
References
- ↑ Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12(18):2249-70. PMID:16787253
- ↑ Van Gelder P, Dumas F, Bartoldus I, Saint N, Prilipov A, Winterhalter M, Wang Y, Philippsen A, Rosenbusch JP, Schirmer T. Sugar transport through maltoporin of Escherichia coli: role of the greasy slide. J Bacteriol. 2002 Jun;184(11):2994-9. PMID:12003940
- ↑ Basle A, Rummel G, Storici P, Rosenbusch JP, Schirmer T. Crystal structure of osmoporin OmpC from E. coli at 2.0 A. J Mol Biol. 2006 Oct 6;362(5):933-42. Epub 2006 Aug 3. PMID:16949612 doi:10.1016/j.jmb.2006.08.002
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky