We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transmembrane protease serine 2
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
Prostate cancer (PC) is the most common form of cancer found in American men and the second leading cause of cancer death. <ref>DOI 10.4172/1948-5956.1000119</ref> This means that approximately 28.5% of cancers and 3.5% of cancer related deaths in men are due to PC. | Prostate cancer (PC) is the most common form of cancer found in American men and the second leading cause of cancer death. <ref>DOI 10.4172/1948-5956.1000119</ref> This means that approximately 28.5% of cancers and 3.5% of cancer related deaths in men are due to PC. | ||
| - | The most prevalent chromosomal aberration causing this pathology is the fusion of the the promoter of transmembrane protease serine 2 (TMPRSS2) gene and the coding sequence of the erythroblastosis virus E26 (Ets) gene family members. <ref>DOI 10.1126/science.1117679</ref> Ets family members are oncogenic transcription factors. <ref>DOI 10.1038/sj.onc.1201868</ref> Therefore, the fusion of these genes leads to the production of Ets transcription factors under the control of the androgen sensitive promoter elements of TMPRSS2. Specifically, the TMPRSS2-ERG fusion has been identified in approximately 50% of PC cases. <ref>DOI 10.1016/j.ccr.2010.03.018</ref> | + | The most prevalent chromosomal aberration causing this pathology is the fusion of the the promoter of transmembrane protease serine 2 (TMPRSS2) gene and the coding sequence of the erythroblastosis virus E26 (Ets) gene family members. <ref>DOI 10.1126/science.1117679</ref> Ets family members are oncogenic transcription factors. <ref>DOI 10.1038/sj.onc.1201868</ref> Therefore, the fusion of these genes leads to the production of Ets transcription factors under the control of the androgen sensitive promoter elements of TMPRSS2. Specifically, the TMPRSS2-ERG fusion has been identified in approximately 50% of PC cases, responsible for driving carcinogenesis. <ref>DOI 10.1016/j.ccr.2010.03.018</ref> |
This mutation occurs through chromosomal translocation or intergenic deletion, with both genes on the same arm of chromosome 21, and results in overexpression of chimeric mRNA of ERG in response to androgens. There is impairment of apoptosis in TMPRSS2-ERG positive cancer cells, possibly due to disruption of the intracellular death domain or decoy receptors. <ref>DOI 10.1186/1475-2867-14-34 </ref> | This mutation occurs through chromosomal translocation or intergenic deletion, with both genes on the same arm of chromosome 21, and results in overexpression of chimeric mRNA of ERG in response to androgens. There is impairment of apoptosis in TMPRSS2-ERG positive cancer cells, possibly due to disruption of the intracellular death domain or decoy receptors. <ref>DOI 10.1186/1475-2867-14-34 </ref> | ||
| Line 27: | Line 27: | ||
=== Viral entry === | === Viral entry === | ||
| - | '''TMPRSS2''' facilitates the entry of viruses into host cells by proteolytically cleaving and activating viral envelope glycoproteins. As human TMPRSS2 is expressed in cells of the respiratory tracts, in addition to the epithelia of the gastrointestinal and urogenital systems, it mediates the entry of several viruses related to respiratory diseases into the host cells, including Influenza virus and the human coronaviruses HCoV-229E, MERS-CoV, SARS-CoV and SARS-CoV-2 (COVID-19 virus). | + | '''TMPRSS2''' facilitates the entry of viruses into host cells by proteolytically cleaving and activating viral envelope glycoproteins (viral spike protein). As human TMPRSS2 is expressed in cells of the respiratory tracts, in addition to the epithelia of the gastrointestinal and urogenital systems, it mediates the entry of several viruses related to respiratory diseases into the host cells, including Influenza virus and the human coronaviruses HCoV-229E, MERS-CoV, SARS-CoV and SARS-CoV-2 (COVID-19 virus). |
====SARS-CoV-2==== | ====SARS-CoV-2==== | ||
Revision as of 21:59, 29 November 2021
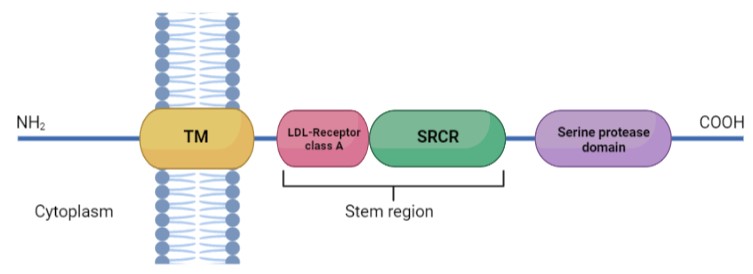
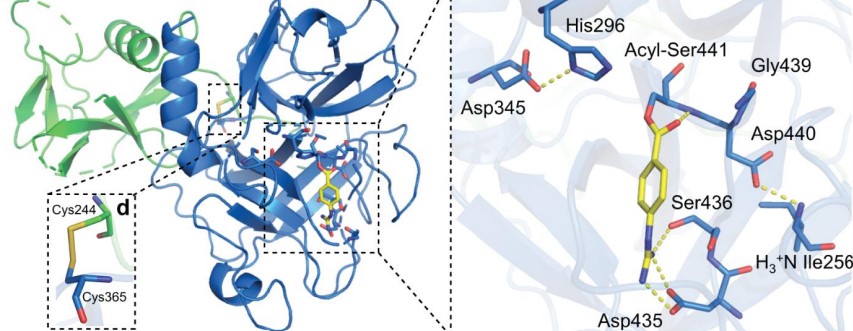
TMPRSS2 is a membrane protein belonging to the type II transmembrane serine protease (TTSP) family. It is functionally classified as a trypsin-like protease (TLP). [1] Serine proteases are known to be involved in many physiological and pathological processes.
| |||||||||||
References
- ↑ Sgrignani J, Cavalli A. Computational Identification of a Putative Allosteric Binding Pocket in TMPRSS2. Front Mol Biosci. 2021 Apr 30;8:666626. doi: 10.3389/fmolb.2021.666626., eCollection 2021. PMID:33996911 doi:http://dx.doi.org/10.3389/fmolb.2021.666626
- ↑ Evnin LB, Vasquez JR, Craik CS. Substrate specificity of trypsin investigated by using a genetic selection. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6659-63. doi: 10.1073/pnas.87.17.6659. PMID:2204062 doi:http://dx.doi.org/10.1073/pnas.87.17.6659
- ↑ Singh N, Decroly E, Khatib AM, Villoutreix BO. Structure-based drug repositioning over the human TMPRSS2 protease domain: search for chemical probes able to repress SARS-CoV-2 Spike protein cleavages. Eur J Pharm Sci. 2020 Oct 1;153:105495. doi: 10.1016/j.ejps.2020.105495. Epub, 2020 Jul 28. PMID:32730844 doi:http://dx.doi.org/10.1016/j.ejps.2020.105495
- ↑ Lam DK, Dang D, Flynn AN, Hardt M, Schmidt BL. TMPRSS2, a novel membrane-anchored mediator in cancer pain. Pain. 2015 May;156(5):923-930. doi: 10.1097/j.pain.0000000000000130. PMID:25734995 doi:http://dx.doi.org/10.1097/j.pain.0000000000000130
- ↑ St John J, Powell K, Conley-Lacomb MK, Chinni SR. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J Cancer Sci Ther. 2012 Apr 26;4(4):94-101. doi: 10.4172/1948-5956.1000119. PMID:23264855 doi:http://dx.doi.org/10.4172/1948-5956.1000119
- ↑ Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005 Oct 28;310(5748):644-8. doi: 10.1126/science.1117679. PMID:16254181 doi:http://dx.doi.org/10.1126/science.1117679
- ↑ Carrere S, Verger A, Flourens A, Stehelin D, Duterque-Coquillaud M. Erg proteins, transcription factors of the Ets family, form homo, heterodimers and ternary complexes via two distinct domains. Oncogene. 1998 Jun 25;16(25):3261-8. doi: 10.1038/sj.onc.1201868. PMID:9681824 doi:http://dx.doi.org/10.1038/sj.onc.1201868
- ↑ Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010 May 18;17(5):443-54. doi: 10.1016/j.ccr.2010.03.018. PMID:20478527 doi:http://dx.doi.org/10.1016/j.ccr.2010.03.018
- ↑ Farooqi AA, Hou MF, Chen CC, Wang CL, Chang HW. Androgen receptor and gene network: Micromechanics reassemble the signaling machinery of TMPRSS2-ERG positive prostate cancer cells. Cancer Cell Int. 2014 Apr 17;14:34. doi: 10.1186/1475-2867-14-34. eCollection, 2014. PMID:24739220 doi:http://dx.doi.org/10.1186/1475-2867-14-34
- ↑ Thunders M, Delahunt B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J Clin Pathol. 2020 Dec;73(12):773-776. doi: 10.1136/jclinpath-2020-206987. Epub , 2020 Sep 1. PMID:32873700 doi:http://dx.doi.org/10.1136/jclinpath-2020-206987
- ↑ Amraei R, Rahimi N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells. 2020 Jul 9;9(7). pii: cells9071652. doi: 10.3390/cells9071652. PMID:32660065 doi:http://dx.doi.org/10.3390/cells9071652
- ↑ doi: https://dx.doi.org/10.1101/2021.06.23.449282
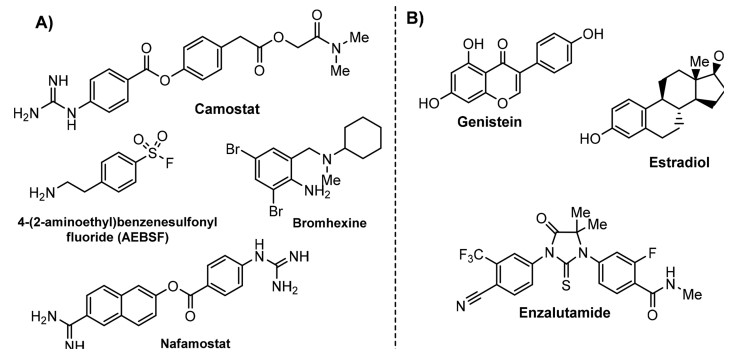
- ↑ Shrimp JH, Kales SC, Sanderson PE, Simeonov A, Shen M, Hall MD. An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19. bioRxiv. 2020 Aug 6. doi: 10.1101/2020.06.23.167544. PMID:32596694 doi:http://dx.doi.org/10.1101/2020.06.23.167544
- ↑ Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985;15(3):164-8. doi: 10.1159/000215139. PMID:3161808 doi:http://dx.doi.org/10.1159/000215139
- ↑ Maggio R, Corsini GU. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol Res. 2020 Jul;157:104837. doi: 10.1016/j.phrs.2020.104837. Epub 2020, Apr 22. PMID:32334052 doi:http://dx.doi.org/10.1016/j.phrs.2020.104837
- ↑ doi: https://dx.doi.org/10.20944/preprints202003.0360.v2
Proteopedia Page Contributors and Editors (what is this?)
Paula R. Mallavibarrena, Ines Muniesa-Martinez, Michal Harel, Laura Aleixos Juan, Jaime Prilusky