Introduction
The major allergen in cats, Fel d 1, belongs to the secretoglobin family of proteins and is, worldwide, one of the major causes of allergic asthma, as it induces IgE responses in 90 to 95% of those allergic to cats and in 60 to 90% of total allergenic activity to cat hair. Symptoms range from mild rhinitis and conjunctivitis to life-threatening asthmatic responses. The structure of Fel d 1 displays the location of three previously defined Fel d 1 IgE epitopes on the surface of the protein [1].
It is produced mainly by the salivary and sebaceous glands, it is also present in the perianal, lacrimal and squamous epithelial cells and is attached to the cat's hair through the habit of licking [1][2].
Function
The biological function of Fel d 1 is still unknown, however it is thought to have a pheromone/chemical signaling function[2].
Structure
Fel d 1 is a tetrameric glycoprotein of 35 to 39 kDa, by size exclusion chromatography, formed by two identical heterodimers of about 18 kDa, noncovalently linked. These heterodimers are totally α-helical, formed by 8 helices, H1-H4 and H5-H8, corresponding to the 2 and 1 chains, respectively[1][3]. Chains 1 and 2 are two antiparallel polypeptides, linked via 3 interchain disulfide bonds, formed between cysteine residues at positions Cys3-Cys73, Cys44-Cys48, and Cys70-Cys7, at chains 1 and 2, respectively [1][3]. Chain 1 has about 8kDa, composed of a residue of 70 amino acids and chain 2 has about 10 kDa, which can be composed of a residue of 90 amino acids, found preferably in the sebaceous glands, or composed of a residue of 92 amino acids, which is expressed by the salivary glands. Its glycan portion is found in chain 2 and the recombinant structure of Fel d 1 reveals that the N33 residue is located in the loop connecting the H2 and H3 helices and that the side chain is exposed to the solvent[1][3].
Figure 1 shows the general structure of a Fel d 1 monomer, shown in two different orientations, rotated 90° around the vertical axis. Chains 1 and 2 correspond to the orange and blue helices respectively. The dotted line indicates the disordered loop (residues 75 to 92). The three disulfide bridges connecting chains 1 and 2 are shown in green. An arrow indicates the unique glycosylation site at residue N33[1].
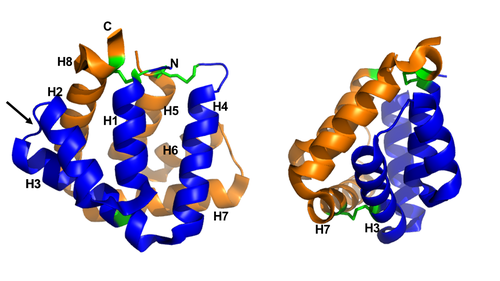
Figure 1. General structure of a Fel d 1 monomer, shown in two distinct orientations, rotated 90° about the vertical axis.
In the Fel d 1 tetramer, three Ca2+ binding sites were identified[3], as in Figure 2, where the Ca2+ are indicated as red balls. Two Ca2+ binding sites are equivalent and are found symmetrically located on either side of the dimer and the third is found within the dimerization interface (Figure 2 (a) and (b)). The Ca2+ equivalents bind to the carbonyl groups of Asp46 and Met49 residues, as well as to four and three water molecules in the A and B subunits, respectively, (Figure 2 (c)) and the Ca2+ located at the dimerization interface binds to the OD1 atoms of residues Asn89 (in subunit A), Asn89 (B) and Asp130 (B), as well as to the carbonyl group of residue Ile125 (A) and three molecules of water (Figure 2 (d))[4].
Figure 2. General structure of the Fel d 1 tetramer. (a) Schematic view of the Fel d 1 tetramer. The two heterodimeric subunits A and B, each composed of the linked 1 and 2 chains, that form the tetramer are yellow and blue, respectively. The three Ca
2+ ions are indicated as red balls. (b) Schematic view of the Fel d 1 tetramer following an approximately 90° rotation about the horizontal axis. (c) Calcium binding sites 1 and 2. (d) Calcium binding site 3
[4].
The Fel d 1 quaternary structure reports two cavities in the A and B subunits of 350 and 730 Å3, respectively, where the difference in size between the two cavities is a direct result of the conformational change within the region corresponding to residues 121– 131. This size difference is related to the conformation of residues Leu129 and Asp130, which point to opposite uptake, in subunit A they point to the cavity and in subunit B they point to a dimerization interface. Furthermore, the Ca2+ from the dimerization interface does not interact with the side chain of the Asp130 from the A subunit, projecting itself, then into its cavity, while the Asp130 side chain of the B subunit interacts with Ca2+. Such cavities have 3 and 7 water molecules, respectively. All of this can be seen in Figure 3[4].
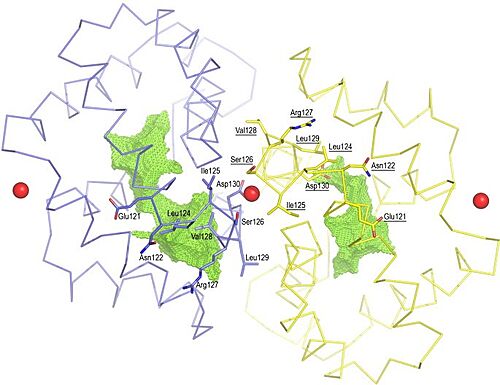
Figure 3. Shape of the cavities (in green) directly governed by the conformation of Leu129 and Asp130 residues. The Asp130 side chain (underlined) does not interact with Ca
2+ and projects into the cavity in the A subunit (in yellow), while the Asp130 side chain (B subunit) binds to Ca
2+. The two external Ca
2+ ions are also indicated
[4].
In the 3D scene of the asymmetric unit of Fel d 1 it is possible to by group, make of the protein Fel d 1 and visualize four MPD molecules (used in the crystallization of Fel d 1) [1] [5] [6].
Treatment
Immunotherapy, or allergy vaccination, which is based on repeated subcutaneous injections of cat hair extracts has been shown to be effective in the curative treatment of allergy. However, in addition to being a time-consuming treatment, it can cause serious side effects such as asthma attacks and anaphylactic shock [1][3].
Additionally, to add to the immunotherapy treatment, there is a cat food supplemented with anti-Fel d 1 IgY, which significantly reduces active Fel d 1 levels. Figure 4 shows the three-dimensional configuration of Fel d 1 (a) and the structure of Fel d 1 linked to two anti-Fel d 1 IgY antibodies (b) [2].
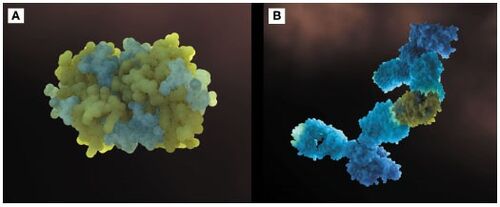
Figure 4. (a) Three-dimensional configuration of Fel d 1 (b) Fel d 1 linked to two anti-Fel d 1 IgY antibodies
[2].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Kaiser L, Gronlund H, Sandalova T, Ljunggren HG, van Hage-Hamsten M, Achour A, Schneider G. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J Biol Chem. 2003 Sep 26;278(39):37730-5. Epub 2003 Jul 8. PMID:12851385 doi:10.1074/jbc.M304740200
- ↑ 2.0 2.1 2.2 2.3 WANDALSEN, Gustavo Falbo; SANO, Flavio; SOLÉ, Dirceu. Alérgenos do gato nas alergias respiratórias: situação atual e novas perspectivas. Asma, alergia e imunologia, São Paulo, SP, Brasil, v. 4, n. 1, p. 61-71, 10 mar. 2020. doi: http://dx.doi.org/10.5935/2526-5393.20200004.
- ↑ 3.0 3.1 3.2 3.3 3.4 GRÖNLUND H, Saarne T, Gafvelin G, van Hage M: The Major Cat Allergen, Fel d 1, in Diagnosis and Therapy. Int Arch Allergy Immunol 2010;151:265-274. doi: 10.1159/000250435.
- ↑ 4.0 4.1 4.2 4.3 KAISER, Liselotte; VELICKOVIC, Tanja Cirkovic; BADIA-MARTINEZ, Daniel; ADEDOYIN, Justus; THUNBERG, Sarah; HALLÉN, Dan; BERNDT, Kurt; GRÖNLUND, Hans; GAFVELIN, Guro; HAGE, Marianne van; ACHOUR, Adnane. Structural Characterization of the Tetrameric form of the Major Cat Allergen Fel d 1. Journal of Molecular Biology, [s. l.], v. 370, ed. 4, p. 714-727, 2007. doi: https://doi.org/10.1016/j.jmb.2007.04.074.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644



