User:Maria Carolina Boer Copstein/Sandbox 1
From Proteopedia
| Line 21: | Line 21: | ||
== Neurodegenerative disease == | == Neurodegenerative disease == | ||
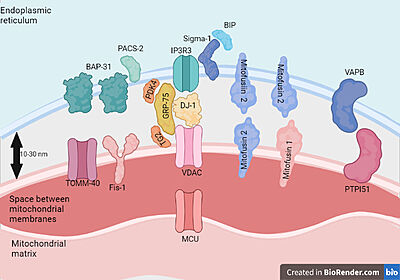
| - | [[Image:ALS dysfunctions.png|500px|right|thumb| | + | [[Image:ALS dysfunctions.png|500px|right|thumb| Alterations in VDAC1 observed in ALS[2].]] |
Several studies have already observed the participation of VDAC1 in mitochondrial dysfunctions observed in several neurodegenerative diseases and constitutes a point of accumulation of protein aggregates with Tau,β amyloid,SOD1 that are present in these pathologies. Due to the involvement of VDAC1 in metabolism and processes of apoptosis this protein becomes a possible therapeutic target of these diseases.The Cu/Zn superoxide dismutase (SOD1) associates with about 20% of familial ALS (fALS) cases and over 180 mutant forms of enzymatically active or inactive SOD1 have been characterized in humans (http://alsod.iop.kcl.ac.uk). In affected tissues, toxic effects of SOD1 mutants are related to the formation of misfolded SOD1 aggregates upon the mitochondrial surface, leading to morphological degeneration and malfunctioning of the organelle.In the spinal cord from ALS patients, voltage dependent anion selective channel isoform 1 (VDAC1) represents the docking site on the outer mitochondrial membrane for ALS-linked SOD1 mutants.In ALS, the VDAC1-SOD1 mutant interaction strongly affects the functional properties of VDAC1 | Several studies have already observed the participation of VDAC1 in mitochondrial dysfunctions observed in several neurodegenerative diseases and constitutes a point of accumulation of protein aggregates with Tau,β amyloid,SOD1 that are present in these pathologies. Due to the involvement of VDAC1 in metabolism and processes of apoptosis this protein becomes a possible therapeutic target of these diseases.The Cu/Zn superoxide dismutase (SOD1) associates with about 20% of familial ALS (fALS) cases and over 180 mutant forms of enzymatically active or inactive SOD1 have been characterized in humans (http://alsod.iop.kcl.ac.uk). In affected tissues, toxic effects of SOD1 mutants are related to the formation of misfolded SOD1 aggregates upon the mitochondrial surface, leading to morphological degeneration and malfunctioning of the organelle.In the spinal cord from ALS patients, voltage dependent anion selective channel isoform 1 (VDAC1) represents the docking site on the outer mitochondrial membrane for ALS-linked SOD1 mutants.In ALS, the VDAC1-SOD1 mutant interaction strongly affects the functional properties of VDAC1 | ||
channel suggesting a role in the impairment of the bioenergetics metabolism and oxidative stress of | channel suggesting a role in the impairment of the bioenergetics metabolism and oxidative stress of | ||
Revision as of 08:27, 13 December 2021
| |||||||||||
References
Magri Andrea ,Messina Angela, “Interactions of VDAC with Proteins Involved in Neurodegenerative Aggregation: An Opportunity for Advancement on Therapeutic Molecules”, Current Medicinal Chemistry 2017; 24(40) . https://doi.org/10.2174/0929867324666170601073920
Hosaka T, Okazaki M, Kimura-Someya T, et al. Crystal structural characterization reveals novel oligomeric interactions of human voltage-dependent anion channel 1. Protein Sci. 2017;26(9):1749-1758. https://doi.org/10.3390/antiox9121218
Pittalà, M.G.G.; Reina, S.; Cubisino, S.A.M.; Cucina, A.; Formicola, B.; Cunsolo, V.; Foti, S.; Saletti, R.; Messina, A. Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation. Antioxidants 2020, 9, 1218. https://doi.org/10.3390/antiox9121218
![Alterations in VDAC1 observed in ALS[2].](/wiki/images/thumb/e/e1/ALS_dysfunctions.png/500px-ALS_dysfunctions.png)
