We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1702
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
===Overall Structure=== | ===Overall Structure=== | ||
===Inactive=== | ===Inactive=== | ||
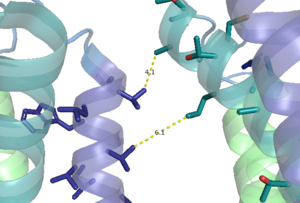
| - | [[Image:TM4_hydrophobic_interactions.png|300 px|left|thumb|Figure 1. Hydrophobic interactions of transmembrane helices III and IV that stabilize the inactive form of mGlu2.]] | + | [[Image:TM4_hydrophobic_interactions.png|300 px|left|thumb|'''Figure 1.''' Hydrophobic interactions of transmembrane helices III and IV that stabilize the inactive form of mGlu2.]] |
===Positive Allosteric Modulator(PAM) Bound=== | ===Positive Allosteric Modulator(PAM) Bound=== | ||
===Negative Allosteric Modulator(NAM) Bound=== | ===Negative Allosteric Modulator(NAM) Bound=== | ||
===Active=== | ===Active=== | ||
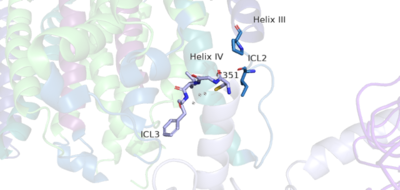
| + | [[Image:Newly labled hook region.png|400 px|left|thumb|'''Figure 5.''' The hook-like region is made up of the last 4 residues on the alpha subunit of the G-protein. Residue C351 hydrophobically interacts with intracellular loop 2 and helix IV. Due to these interactions, the G-protein is able to bind to a shallow groove formed by intracellular loops 2 and 3. ]] | ||
==Clinical Relevance== | ==Clinical Relevance== | ||
Revision as of 14:31, 22 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Metabotropic Glutamate Receptor 2
| |||||||||||