We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1725
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
===Catalytic Cysteines=== | ===Catalytic Cysteines=== | ||
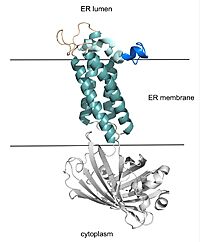
| - | VKOR uses four catalytic cysteines (43, 51, 132, and 135) to facilitate reduction and cause conformational changes via disulfide bridge formation. When an oxidized or partially oxidized Vitamin K enters the active site, VKOR has a stabilizing C51-C132 disulfide bond. C135 is not | + | VKOR uses four catalytic cysteines (43, 51, 132, and 135) to facilitate reduction and cause conformational changes via disulfide bridge formation. When an oxidized or partially oxidized Vitamin K enters the active site, VKOR has a stabilizing C51-C132 disulfide bond, known as the closed state. C135 is not in a disulfide bridge and is important in helping Vitamin K bind. C43 attacks the C51-C132 bond, forming a new C43-C51 bond that characterizes the open state. The free amino acid at the 132 position is shown as a serine in this depiction because it was mutated it to stop the reaction at this intermediate during Liu's experimental procedure. <ref name="Liu">PMID:33154105</ref>. In the wild type VKOR, the 132 position would be a cysteine. C132 forms a bridge with C135 which allows release of the reduced or partially reduced Vitamin K. These rearrangements facilitate the use of cysteines as reducing agents for Vitamin K. Cysteine rearrangements also contribute to and are affected by overall conformational changes which affects their proximity to each other and the active site. |
| - | + | Warfarin binding also depends on the catalytic cysteines. Warfarin is able to bind to the fully oxidized form of VKOR where the disulfide bridge pairings are C132-C135 and C43-C51. Warfarin can also bind to the partially oxidized form of VKOR where the disulfide bridge pairings are the same as the closed state, with C51-C132 being the only bridge. Again, C43 is shown as a serine because a mutation was used to force VKOR to adopt that conformation during the experimental procedure by Liu. <ref name="Liu">PMID:33154105</ref>. | |
| - | + | ||
Revision as of 20:49, 24 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
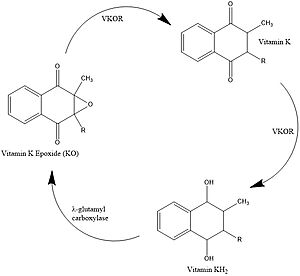
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 2.2 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
Student Contributors
Izabella Jordan, Emma Varness