We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1713
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
==Introduction== | ==Introduction== | ||
| - | Anaplastic Lymphoma Kinase is a receptor tyrosine [https://pubmed.ncbi.nlm.nih.gov/32114309/ kinase]. ALK transfers a phosphate group from [https://www.ncbi.nlm.nih.gov/books/NBK553175/ ATP] to a tyrosine residue on an enzyme which activates a signaling cascade, and ALK becomes activated when a ligand called [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789956/ ALKAL] binds to the binding surface on an extracellular domain of ALK | + | Anaplastic Lymphoma Kinase is a receptor tyrosine [https://pubmed.ncbi.nlm.nih.gov/32114309/ kinase] and is an integral membrane protein. ALK transfers a phosphate group from [https://www.ncbi.nlm.nih.gov/books/NBK553175/ ATP] to a tyrosine residue on an enzyme which activates a signaling cascade, and ALK becomes activated when a ligand called [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789956/ ALKAL] binds to the binding surface on an extracellular domain of ALK. |
| - | == Function == | + | == Structure & Function == |
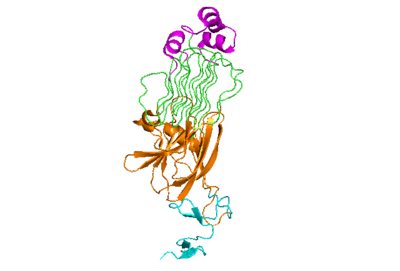
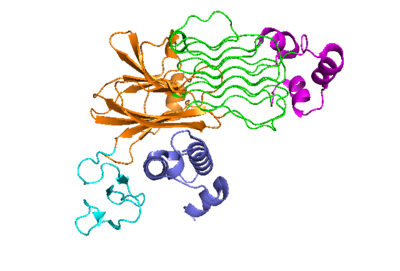
| + | The extracellular portion of ALK has an inactive state, which is its monomerized form, and an active dimerized state with its ligands bound. The monomer is shown to the right in Figure 1, which has many different domains. The growth factor-like domain (EGF) connects the extracellular domains to the transmembrane domain (cyan). The tumor necrosis factor-like domain (TNFL) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand(orange). The glycine-rich domain (GlyR) contains 14 rare polyglycine helices that are hydrogen-bound to each other (green). The <scene name='90/904318/Glycinerichdomain/1'>hexagonal orientation</scene> of these rare helices create a very rigid structure that is important for ALK function. The polyglycine extension loop (PXL) connects two of these polyglycine helices. | ||
[[Image:Prote_ALK_Monomer_White.png|400 px|right|thumb|Figure 1]] | [[Image:Prote_ALK_Monomer_White.png|400 px|right|thumb|Figure 1]] | ||
[[Image:Proteo_ALK-ALKAL_Monomer_White.png|400 px|right|thumb|Figure 2]] | [[Image:Proteo_ALK-ALKAL_Monomer_White.png|400 px|right|thumb|Figure 2]] | ||
<scene name='90/904318/Binding_surface_with_residues/3'>interacting residues of ALK and ALKAL</scene> | <scene name='90/904318/Binding_surface_with_residues/3'>interacting residues of ALK and ALKAL</scene> | ||
<scene name='90/904318/Alk-alkal_binding_surface/2'>ALK-ALKAL binding surface</scene> | <scene name='90/904318/Alk-alkal_binding_surface/2'>ALK-ALKAL binding surface</scene> | ||
| - | <scene name='90/904318/Glycinerichdomain/1'>structure of the glycine-rich domain</scene> | ||
<scene name='90/904318/Alkalbindingsurfacewmembrane/1'>Binding surface of ALKAL with the membrane</scene> | <scene name='90/904318/Alkalbindingsurfacewmembrane/1'>Binding surface of ALKAL with the membrane</scene> | ||
<scene name='90/904318/Alkal1membraneinteraction/2'>ALKAL's residues that interact with membrane</scene> | <scene name='90/904318/Alkal1membraneinteraction/2'>ALKAL's residues that interact with membrane</scene> | ||
Revision as of 02:39, 28 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase
| |||||||||||
References
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8