Introduction
Neurofibromatosis Type 1 is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the GTPase-activating protein neurofibromin. Neurofibromin is closely involved in signaling pathways such as MARP/ERK, P13K/AKT/mTOR, and other cell signaling pathways that use Ras [1]. Mutations that cause a decrease in activity of neurofibromin cause tumors to grow along your nerves. As neurofibromin is ubiquitously expressed throughout the body, these tumors can grow anywhere. Neurofibromin is located in the cytosol of the cell but is recruited to the plasma membrane to bind to Ras. The structure of neurofibromin was detemed through high-resolution single particles cryogenic electron microscopy to understand the overall structure and the different domains. X-Ray crystallography experiments found inconsistency in the structural determination.[1][2][3][4]
Function
Neurofibromin is a GTPase-activating protein that binds to Ras, a GTPase, to increase the hydrolysis of GTP to GDP. This inactivates the cell signaling of Ras until another GTP can replace the GDP from the cytosol. Neurofibromin and Ras binding is possible in only the of Neurofibromin. The mechanism is shown in figure 1 and displays the slow hydrolysis of GTP bound to Ras and the fast hydrolysis of GTP when bound to Neurofibromin. [2][3][4]
Structure
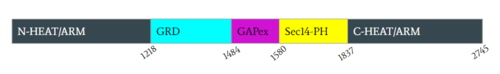
Neurofibromin is a protein dimer that exists in the and conformation. Each protomer contains a GRD, Sec14-PH, and a GAPex domain located on a HEAT N-C arm. Ras binds to the GRD site with Arg1276 being the critical residue for binding. [2][3][4]
Closed conformation
Ras is unable to bind to the GRD active site when both of the NF1 protomers are in the closed confirmation. In the, one protomer has its domains shifted by a 130 degree rotation of three separate linkers. That rotation places in an orientation that in the GRD site (Figure 3). Also in this depiction Ras binding to the GRD site is inhibited by the N-HEAT ARM as it interferes at the GRD in Ras attempting to bind to the active site and is sterically hindered. Making sterically impossible in the
. The can exist naturally without any form of stabilization but will also fall back to the .[2] [3][4]
Zinc Stabilized
The of Neurofibromin can be stabilized by a zinc ion to prevent the shift back to an . This binding is done between C1032, H1558, and H1576 within the N-HEAT domain, GRD-Sec14-PH linker L2 and is shown in figure #. When zinc stabilizes Neurofibromin, it will stay in the and continue to inhibit the binding of RAS.[2][3][4]
Open conformation
In the open state, one of the protomers remains in the closed confirmation inaccessible to Ras, while the other protomer is conformationally changed into the open form with Ras bound.
In the the one protomer is shifted due to a 90 rotation. This rotation allows for binding between RAS and the
in the GRD site while in the . Allowing for the to occur without any steric hindrance as shown in the . In the open state, one of the protomers remains in the closed confirmation inaccessible to Ras, while the other protomer is conformationally changed into the open form with Ras bound. It is important to mention, the GRD and Sec14-PH are reoriented away from one another and the GRD site is no longer sterically hindered and clashing with the N-HEAT ARM and is completely accessible for Ras to bind. To undergo the movement to the open confirmation, significant conformational changes exist within three separate linkers (L1, L2, L3).[2] [3][4]
Conformational Change Linkers
The rotation of the domains between the and of Neurofibromin are conducted by three helical linkers named L1, L2, and L3. The
and the
undergo rotations to relocate the GRD and Sec14-PH sites. Linker 1 (L1) consists of a loop connected by two helices from L1173-M1215 and is the main contributor in rotation of the GRD domain. The rotation of L1 to the causes N-HEAT ARM alpha helix 48 and GRD helix 49 to extend out, aligning to form a hinge point at G1190. The GRD relocation is assisted by Sec14-PH relocation, which is initiated by Linker 3(L3) from Q1835 to G1852 where the proline rich section of the C-HEAT ARM changes conformation.L1 and L3 move closer to each other in the allowing for the GRD and Sec14-PH domain to initiate their significant rearrangement. Linker 2 (L2) consists of residues G1547-T1565 and begins at helix 63, the final helix of the GRD site, and connects into the short loop of alpha helix 65 of the Sec14-PH domain and also assists in shifting the Sec14-PH away from the GRD site. The combination of these three linkers are responsible for the conformational shift of the
and
.[3][4]
Domains
N-C HEAT ARM
NF1 in its closed and open confirmations holds five separate domains. The first domain shown in black is representative of two separate but very similar domains, the N-HEAT ARM(N-Terminal) and C-HEAT ARM(C-Terminal). Structurally the N-HEAT Arm’s consists of various helices and loops that interconnect on one of the existing protomers. The N-HEAT ARM is critical in stabilization and linking the other domains which extend out from the core and are important to its catalytic function. The N-HEAT ARM in its linkage with the GRD catalytic domain, has a direct impact on the relative conformational change from the active (Ras BOUND) to inactive (Ras unbound) states. Finally, the Zn2+ binding site also exists downstream on the N-HEAT ARM just before the GRD and SEC14-PH domains. The C-HEAT Arm is linked structurally to the N-HEAT ARM and is a continuation of the protomer. It extends out on the opposing end from the other domains. In its structure consisting of various loops and helices, it is involved with stabilization and linking to the GRD and SEC14-PH and plays a role in the conformational changes from the closed to open state.[3][4]
GRD
Neurofibromin’s main catalytic domain is the GRD active site. Linked structurally to both HEAT ARM’s, it consists of mainly loops and helices. Per protomer, there is one single GRD binding site. In the closed state Ras cannot bind due to a steric hindrance in which Ras clashes with the N-HEAT ARM upon attempting to bind to the GRD site. In its active state GRD can bind Ras. The critical residue within the GRD site is Arg1276.[3][4]
GAPex-Subdomain
The GAPex subdomain of the GRD site lies between the Sec14-PH and GRD catalytic sites. This domain is non-catalytic and structurally consists of various loops and helices. Its main function is to bind SPRED-1, which is a recruiter protein that binds to this subdomain in the cytosol to recruit Neurofibromin to the plasma membrane. [3][4]
Sec14-PH
The Sec14-PH domain is linked and extends out from the HEAT ARM’s and consists of largely various helices and loops. Its function is a membrane associated domain and holds a largely hydrophobic cavity allowing for binding to the plasma membrane. In the it is blocked by the GRD and is inaccessible to the lipid membrane. In the it becomes exposed and can access the lipid membrane for interaction.[3][4]
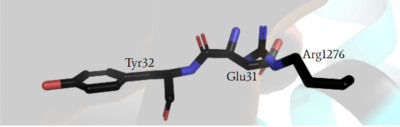
Arginine 1276
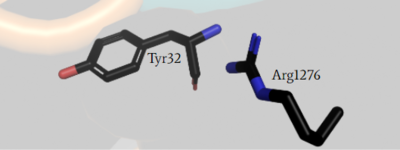
Arg1276 is the critical residue within the GRD site needed for proper binding to Ras. The interaction between
is only possible in the open confirmation (Figure #). In the open confirmation, the arginine finger binds to the backbone gamma carbon of Y32 to assist in eventual hydrolysis of GTP. In the closed conformation this was inhibited by E31 but in the open conformation the conformational change allows for an interaction of R1276 and the gamma carbon so they align with one another. This allows for the normal function of NF1 in the rapid rate increase of GTP hydrolysis upon Ras binding.
has steric clashing with Ras making binding of Ras impossible (Figure #). In the closed confirmation the Arginine finger attempts to bind the backbone gamma carbon(RAS) of a Tyrosine residue(Y32) to assist in hydrolysis, but instead clashes with a Glutamate residue(E31) that would otherwise not interfere in the open conformation and can no longer bind properly. This residue impacts binding of Ras and is of critical importance in determining if Ras is able to bind to the GRD site or not to assist in eventual hydrolysis of GTP.
Mutations to the arginine finger are shown to slow function of NF1 and other GTPases in their function in accelerating GTP hydrolysis. When a Ras GAP such as NF1 can functionally utilize its arginine finger, it allows NF1 to rapidly hydrolyze GTP when bound to Ras. This emphasizes the importance of the R1276 residue for the GRD site binding to Ras.
[2][3][4]
SPRED 1
SPRED 1 is a protein that binds to the GAPex domain of Neurofibromin. Its function recruits the Neurofibromin protein when bound from the cytosol to the plasma membrane. SPRED 1 will bind to the GAPex domain of Neurofibromin in the in the cytosol to recruit Neurofibromin to the plasma membrane. Unlike Ras, SPRED-1 does show the ability to . When bound to the open conformation of Neurofibromin in the cytosol, it may present a different orientation that impacts the recruitment to the plasma membrane. Further research is needed to assess the impact of the function and the changes it may present.[3][4]
Future Clinical Relevance
Germline mutations are common in NF1, often causing genetic tumor syndrome through misregulation of the Ras signaling pathway. Somatic mutations among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, clinical findings show tumors that develop often along the deep epidermis layer of the skin. The most common among NF1 mutations is Neurofibroma. Physically, soft skin-colored pink papules develop on the extremities or on the neck. Also in patients they become prevalent during puberty. Among germline mutations Neurofibroma seems to be the most well known, other tumors may also be common. Lisch Nodules are also common in the development of melanoma within melanocytes as a result of NF1 mutations. Plexiform Neurofibroma and Optic Glioma also are tumor based syndromes resulting from these germline mutations. Increased risk in cancer for patients with mutations to NF1 is about 4 fold greater. Treatment options do exist for patients involving a multitude of drug based treatments coupled with chemotherapy. Surgery and laser treatments are also common.
Mutations to NF1 in the critical interaction involved in the GRD active site with R1276, structurally and functionally prove why they can cause cancer, as Ras is no longer mediated. The importance of this residue when binding to Ras may be able to contribute to future studies in potentially mimicking this arginine finger when bound to Ras, in cases where mutations would impact R1276 in its normal function. Observing the nature of NF1 can further drug development and further improve what is already known about NF1.[1][5][6]
References placeholders
[1]
[2]
[5]
[3]
[4]
[6]
References
- ↑ 1.0 1.1 1.2 1.3 Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Bourne HR. G proteins. The arginine finger strikes again. Nature. 1997 Oct 16;389(6652):673-4. doi: 10.1038/39470. PMID:9338774 doi:http://dx.doi.org/10.1038/39470
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ 5.0 5.1 Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest. 2017 Feb;97(2):146-157. doi: 10.1038/labinvest.2016.142. Epub 2017, Jan 9. PMID:28067895 doi:http://dx.doi.org/10.1038/labinvest.2016.142
- ↑ 6.0 6.1 Sabatini C, Milani D, Menni F, Tadini G, Esposito S. Treatment of neurofibromatosis type 1. Curr Treat Options Neurol. 2015 Jun;17(6):355. doi: 10.1007/s11940-015-0355-4. PMID:25917340 doi:http://dx.doi.org/10.1007/s11940-015-0355-4