Sandbox Reserved 1703
From Proteopedia
| Line 6: | Line 6: | ||
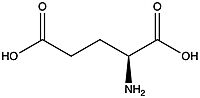
'''Metabotropic glutamate receptors''' are found in the central nervous system and play a critical role in modulating cell excitability and synaptic transmission <ref name="Lin">PMID: 34135510</ref>. Glutamate, shown in Figure 1, is a negatively charged polar amino acid that is the main neurotransmitter in the brain. Glutamate activates 8 different types of metabotropic glutamate receptors<ref name="Seven">Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3</ref>. '''Metabotropic Glutamate Receptor 2 (mGlu2)''' is a member of the [https://en.wikipedia.org/wiki/Class_C_GPCR Class C GPCR]Family and can further be classified into the Group II subgroup of metabotropic receptors. Since mGlu2 is a part of the Class C GPCR family, it undergoes small conformational changes to the transmembrane domain (TMD) to move from the inactive to the fully active structure<ref name="Lin" />. Functionality of mGlu2 will be dependent on the concentration of glutamate. Higher concentrations of glutamate will promote stronger signal transduction from the extracellular domain to the transmembrane domain. [[Image:Glutamate.jpeg|200px|right|thumb|'''Figure 1.'''The structure of glutamate.]] | '''Metabotropic glutamate receptors''' are found in the central nervous system and play a critical role in modulating cell excitability and synaptic transmission <ref name="Lin">PMID: 34135510</ref>. Glutamate, shown in Figure 1, is a negatively charged polar amino acid that is the main neurotransmitter in the brain. Glutamate activates 8 different types of metabotropic glutamate receptors<ref name="Seven">Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3</ref>. '''Metabotropic Glutamate Receptor 2 (mGlu2)''' is a member of the [https://en.wikipedia.org/wiki/Class_C_GPCR Class C GPCR]Family and can further be classified into the Group II subgroup of metabotropic receptors. Since mGlu2 is a part of the Class C GPCR family, it undergoes small conformational changes to the transmembrane domain (TMD) to move from the inactive to the fully active structure<ref name="Lin" />. Functionality of mGlu2 will be dependent on the concentration of glutamate. Higher concentrations of glutamate will promote stronger signal transduction from the extracellular domain to the transmembrane domain. [[Image:Glutamate.jpeg|200px|right|thumb|'''Figure 1.'''The structure of glutamate.]] | ||
| - | mGlu2 plays vital roles in memory formation, pain management, and addiction, which makes it an important drug target for | + | mGlu2 plays vital roles in memory formation, pain management, and addiction, which makes it an important drug target for [https://en.wikipedia.org/wiki/Parkinson%27s_disease Parkinson’s Disease],[https://en.wikipedia.org/wiki/Schizophrenia Schizophrenia], [https://en.wikipedia.org/wiki/Cocaine_dependence cocaine dependence], and many other neurological conditions. |
| + | <ref name="Zhang">Zhang, Zhu, et al. “Roles of Glutamate Receptors in Parkinson's Disease.” MDPI, Multidisciplinary Digital Publishing Institute, 6 Sept. 2019, https://dx.doi.org/10.3390%2Fijms20184391.> | ||
==Structure== | ==Structure== | ||
Revision as of 13:51, 5 April 2022
Metabotropic Glutamate Receptor 2
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3
- ↑ Zhang, Zhu, et al. “Roles of Glutamate Receptors in Parkinson's Disease.” MDPI, Multidisciplinary Digital Publishing Institute, 6 Sept. 2019, https://dx.doi.org/10.3390%2Fijms20184391.>
Structure
Overall Structure
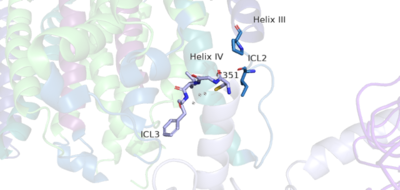
Cryo-EM studies of mGlu2 have yielded adequate structures that have acted as maps to aid in producing a better structural understanding of the inactive and active states of mGlu2<ref></ref>. The overall of the mGlu2 is composed of 3 main parts: a ligand binding , followed by a linker to the Transmembrane Domain that contains on both the chains that aid in the binding of the G-Protein. Class C CPCRs such as mGlu2, are activated by their ability to form dimers. MGlu2 is a homodimer which is imperative to the receptor’s ability to relay signals induced by glutamate from the extracellular domain(ECD) to its transmembrane domain(TMD). The homodimer of mGlu2 contains an alpha chain and a beta chain. Occupation of both ECDs with the agonist, glutamate, is necessary for a fully active mGlu2<ref>Du, Juan, et al. “Structures of Human mglu2 and mglu7 Homo- and Heterodimers.” Nature News, Nature Publishing Group, 16 June 2021, https://www.nature.com/articles/s41586-021-03641-w.></li> <li id="cite_note-Du">[[#cite_ref-Du_0|↑]] <strong class="error">Cite error: Invalid <code><ref></code> tag; no text was provided for refs named <code>Du</code></strong></li> <li id="cite_note-Ellaithy-4">[[#cite_ref-Ellaithy_4-0|↑]] Ellaithy A, Younkin J, Gonzalez-Maeso J, Logothetis DE. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015 Aug;38(8):506-16. doi: 10.1016/j.tins.2015.06.002. Epub, 2015 Jul 4. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/26148747 26148747] doi:[http://dx.doi.org/10.1016/j.tins.2015.06.002 http://dx.doi.org/10.1016/j.tins.2015.06.002]</li>
<li id="cite_note-Muguruza-5">[[#cite_ref-Muguruza_5-0|↑]] Muguruza C, Meana JJ, Callado LF. Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front Pharmacol. 2016 May 20;7:130. doi: 10.3389/fphar.2016.00130. eCollection, 2016. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27242534 27242534] doi:[http://dx.doi.org/10.3389/fphar.2016.00130 http://dx.doi.org/10.3389/fphar.2016.00130]</li></ol></ref>
Student Contributors
Frannie Brewer and Ashley Wilkinson