We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1717
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <scene name='90/904322/Open_conformation/1'>Text To Be Displayed</scene>=Vitamin K Epoxide Reductase= | ||
| - | |||
<StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | <StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | ||
| - | [[Image:VKORimage3.png|250px|right|thumb|Figure 1. Closed Conformation of VKOR due to Warfarin Binding]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
== Introduction == | == Introduction == | ||
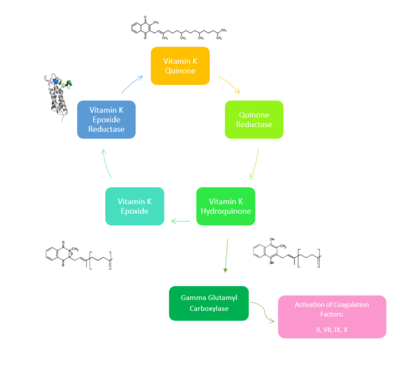
| - | [[Image:NewVitaminKCycle.PNG| | + | [[Image:NewVitaminKCycle.PNG|400px|right|thumb|'''Figure 2. Overview of Vitamin K Cycle''': The cycle begins with [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K Quinone]. Vitamin K Quinone is reduced by enzyme Quinone Reductase. This leaves Vitamin K Hydroquinone which can either lead to [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase Gamma Carboxylase]activity that will activate Blood Coagulation Factors II, VII, IX, and X. After this, Vitamin K Epoxide is left over. Vitamin K Epoxide is reduced by the enzyme Vitamin K Epoxide Reductase to reform Vitamin K Quinone. ]] |
| - | + | <scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | |
| - | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors II, VII, IX, and X. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. | + | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. |
| + | ===Reaction=== | ||
| + | In the Vitamin K Cycle (Fig 1), there are two primary enzymes, the latter enzyme is Vitamin K Epoxide Reductase. Four conserved cysteines: Cys43, Cys51, Cys132, and Cys135 will aid in a redox reaction that will allow for Vitamin K Epoxide to be reduced back to Vitamin K Quinone. VKOR begins and ends in an open conformation and is fully oxidized. The second step is a partially oxidized state where Catalytic Cysteines 51 and 132 share a disulfide bond. No ligand is within the active site at either of these stages. In the third stage Vitamin K Epoxide binds to the active site and VKOR becomes closed and is still partially oxidized. Cysteine 51 and 132 still share a disulfide bond, and Cysteine 135 binds to Vitamin K Epoxide. When Cysteine43 forms a disulfide bond with Cysteine 51, Cysteine 132 will bind to Cysteine 135. Cysteine 135 will form the disulfide bond with Cysteine132 and kick its extra electrons to Vitamin K Epoxide. The transfer of electrons opens up the epoxide ring, which is reforming Vitamin K Quinone. This is another fully oxidized state of VKOR. Then, the cycle restarts at the beginning in an open conformation to release the Vitamin K Quinone. <ref name="Goodstadt">PMID:15276181</ref> | ||
== Structure == | == Structure == | ||
| Line 19: | Line 14: | ||
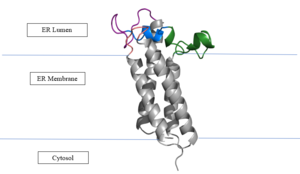
The VKOR enzyme is made up of four transmembrane helices: T1, T2, T3, and T4.(Grey) Each of these helices come together to form a central ligand binding pocket. This central pocket is the active site where conserved Cysteines: C132 and C135 are located. In the cap domain are important regions that are significant for Vitamin K binding, and the overall function of Vitamin K Epoxide Reductase, including the Anchor(Green), Cap Sequence (Blue), Beta Hairpin (Purple), and 3-4 Loop (Pink). | The VKOR enzyme is made up of four transmembrane helices: T1, T2, T3, and T4.(Grey) Each of these helices come together to form a central ligand binding pocket. This central pocket is the active site where conserved Cysteines: C132 and C135 are located. In the cap domain are important regions that are significant for Vitamin K binding, and the overall function of Vitamin K Epoxide Reductase, including the Anchor(Green), Cap Sequence (Blue), Beta Hairpin (Purple), and 3-4 Loop (Pink). | ||
| - | '' | + | The <scene name='90/904321/Anchor/3'>Anchor</scene> attaches to the cap domain of the Vitamin K Epoxide Reductase Enzyme and is partially embedded in the Endoplasmic Reticulum Membrane. This both stabilizes the enzyme in the membrane, and stabilizes the cap domain over the active site. |
| - | '' | + | The <scene name='90/904321/Cap_sequence/1'>Cap Sequence</scene> is two parts: The cap helix and the cap loop. When the enzyme is reducing Vitamin K Epoxide or being inhibited by Vitamin K Antagonists, this cap region swings downward over the active site. The cap region is directly attached to the anchor. |
''' | ''' | ||
| - | '' | + | The <scene name='90/904321/Beta_hairpin/1'>Beta Hairpin</scene> is only seen in the closed conformation of Vitamin K Epoxide Reductase. When in the open conformation the beta hairpin is referred to as the luminal helix (yellow). The Beta hairpin is significant due to the fact that it contains the other two conserved cysteines necessary for the function of Vitamin K Epoxide Reductase: Cysteine43 and Cysteine51. The beta hairpin/luminal helix is directly connected to the cap region. |
| - | '' | + | The <scene name='90/904321/3-4_loop/2'>Loop 3-4</scene> is the sequence of residues between Transmembrane Helix 3 and Transmembrane Helix 4. In the open conformation the loop does not have significant interactions with the rest of the cap domain, however in the closed conformation Loop 3-4 has many hydrogen reactions with the Cap Loop. This allows for the stabilization when VKOR is closed. |
| Line 37: | Line 32: | ||
| + | ===Transmembrane Helices=== | ||
| + | The Transmembrane region has four helices, labeled 1-4. Transmembrane Helix 2 (TM2) and 4 (TM4) contribute to the binding of Vitamin K to the hydrophobic pocket for reduction. Asparagine 83 on TM2 and Tyrosine 142 on TM4 hydrogen bond to Vitamin K Epoxide to hold it in the correct orientation for reduction. The angle in which Vitamin K Epoxide binds in the central pocket is significant to the placement of the beta hairpin, and loop 3-4, Cysteine residues C51-C132 will donate their electrons to Vitamin K Epoxide (Fig. 4) to open the epoxide ring, and reform Vitamin K Quinone (Fig. 2). | ||
| + | ===Cap Domain=== | ||
| + | The cap domain of Vitamin K Epoxide Reductase plays an intricate role in its function. When Vitamin K Epoxide (or a similar substrate) binds in the hydrophobic pocket of VKOR, the cap domain undergoes a conformational change that will allow for specific cysteine residues to be able to open the epoxide ring and to recreate Vitamin K Quinone. The catalytic cycle begins in an open fully oxidized conformation. <scene name='90/904321/Openvkor/3'>Open Oxidized Conformation</scene> This conformation has slightly different parts. These include the Anchor (green), the cap region (blue), 3-4 Loop (pink), and luminal helix (yellow). When Vitamin K Epoxide binds, the entire cap domain undergoes a slight conformation change, but the luminal helix has a larger change. The luminal helix (yellow) bends forward where specific cysteines on this region are in proximity to other important cysteines. The luminal helix is then referred to as the beta hairpin (purple). <scene name='90/904321/Vkoclosed/3'>Partially Oxidized Closed Conformation</scene> | ||
| + | ==Catalytic Cysteines== | ||
| + | A set of four catalytic are required for Vitamin K Epoxide reduction and are consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/904321/Cysteines/6'>Significant Cysteines</scene> In the Pufferfish homolog (TrVKORL) these cysteines, due to Cryo-EM differences,are Cys52, Cys55, Cys141, and Cys144. These cysteines donate their electrons to the epoxide ring on Vitamin K Epoxide which reforms Vitamin K Quinone. In the closed active conformation, that is induced when Vitamin K binds in the hydrophobic pocket. (NEED TO FINISH) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | ===Transmembrane Helices=== | ||
| - | The Transmembrane region has four helices, labeled 1-4. Transmembrane Helix 2 (TM2) and 4 (TM4) contribute to the binding of Vitamin K to the hydrophobic pocket for reduction. Asparagine 83 on TM2 and Tyrosine 142 on TM4 hydrogen bond to Vitamin K Epoxide to hold it in the correct orientation for reduction. The angle in which Vitamin K Epoxide binds in the central pocket is significant to the placement of the beta hairpin, and loop 3-4, Cysteine residues C51-C132 will donate their electrons to Vitamin K Epoxide (Fig. 4) to open the epoxide ring, and reform Vitamin K Quinone (Fig. 2). | ||
| - | |||
| - | ===Cap Domain=== | ||
| - | |||
| - | The cap domain of Vitamin K Epoxide Reductase plays an intricate role in its function. When Vitamin K Epoxide (or a similar substrate) binds in the hydrophobic pocket of VKOR, the cap domain undergoes a conformational change that will allow for specific cysteine residues to be able to open the epoxide ring and to recreate Vitamin K Quinone. The catalytic cycle begins in an open fully oxidized conformation. <scene name='90/904321/Openvkor/3'>Open Oxidized Conformation</scene> This conformation has slightly different parts. These include the Anchor (green), the cap region (blue), 3-4 Loop (pink), and luminal helix (yellow). When Vitamin K Epoxide binds, the entire cap domain undergoes a slight conformation change, but the luminal helix has a larger change. The luminal helix (yellow) bends forward where specific cysteines on this region are in proximity to other important cysteines. The luminal helix is then referred to as the beta hairpin (purple). <scene name='90/904321/Vkoclosed/3'>Partially Oxidized Closed Conformation</scene> | ||
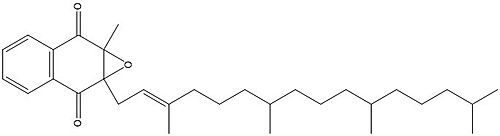
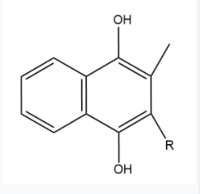
== Vitamin K Epoxide == | == Vitamin K Epoxide == | ||
| Line 70: | Line 49: | ||
[[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 4. Vitamin K Epoxide structure]] | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 4. Vitamin K Epoxide structure]] | ||
| - | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle | + | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. |
Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | ||
| Line 76: | Line 55: | ||
[[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 5. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 6. Vitamin K Hydroquinone structure]] | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 5. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 6. Vitamin K Hydroquinone structure]] | ||
=== Binding === | === Binding === | ||
| - | |||
In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/1'>open conformation</scene>. The Vitamin K epoxide enters through the isoprenyl-chain tunnel. The ketones on the VK epoxide bind to <scene name='90/904322/Vko_binding/1'>Asn80 and Tyr139</scene> on VKOR. With Vitamin K epoxide bound, the cysteines of VKOR are partially oxidized, and concurrently reduce the substrate. A disulfide bond then forms between Cys51 and Cys132, resulting in the closed conformation. This leaves the sulfur on Cys43 and the sulfur on Cys135 protonated. The available hydrogens on these cysteines are utilized in reducing the epoxide. First, the free sulfur on Cys43 attacks Cys51 to form a new disulfide bond. With the loss of hydrogen from Cys43 in the formation of the new disulfide bond, an electron transfer is made to VKO. Next, the sulfur on Cys132 and the sulfur on Cys135 form a new disulfide bond. The hydrogen that was present on Cys135 is lost in the formation of the disulfide bond, allowing for an electron transfer to the oxygen of the epoxide. With these cysteine pairs formed, VKOR is left in an open conformation. The end products are Vitamin K/quinone and water. | In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/1'>open conformation</scene>. The Vitamin K epoxide enters through the isoprenyl-chain tunnel. The ketones on the VK epoxide bind to <scene name='90/904322/Vko_binding/1'>Asn80 and Tyr139</scene> on VKOR. With Vitamin K epoxide bound, the cysteines of VKOR are partially oxidized, and concurrently reduce the substrate. A disulfide bond then forms between Cys51 and Cys132, resulting in the closed conformation. This leaves the sulfur on Cys43 and the sulfur on Cys135 protonated. The available hydrogens on these cysteines are utilized in reducing the epoxide. First, the free sulfur on Cys43 attacks Cys51 to form a new disulfide bond. With the loss of hydrogen from Cys43 in the formation of the new disulfide bond, an electron transfer is made to VKO. Next, the sulfur on Cys132 and the sulfur on Cys135 form a new disulfide bond. The hydrogen that was present on Cys135 is lost in the formation of the disulfide bond, allowing for an electron transfer to the oxygen of the epoxide. With these cysteine pairs formed, VKOR is left in an open conformation. The end products are Vitamin K/quinone and water. | ||
| - | ===Catalytic Cysteines=== | ||
| - | |||
| - | A set of four cysteines is consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/904321/Cysteines/6'>Significant Cysteines</scene> In the Pufferfish homolog (TrVKORL) these cysteines, due to Cryo-EM differences,are Cys52, Cys55, Cys141, and Cys144. These cysteines are the key factor that allow for Vitamin K Epoxide Reductase to perform its function, which is to open the epoxide ring on Vitamin K Epoxide in order to re-make Vitamin K Quinone. In the closed conformation, that is induced when Vitamin K binds in the hydrophobic pocket, Cys-132 binds to Cys-51 and Cys-135 will bind to the 3' hydroxyl group on Vitamin K Epoxide, which allows for the electron transfer to open up the epoxide ring. <scene name='90/904321/Cys52disulfidecys55/9'>Electron Transfer through Cysteine138 TrVKORL</scene> | ||
| Line 98: | Line 73: | ||
=== Disease === | === Disease === | ||
| - | Vitamin K | + | [https://en.wikipedia.org/wiki/Vitamin_K_antagonist#:~:text=Vitamin%20K%20antagonists%20(VKA)%20are,the%20recycling%20of%20vitamin%20K. Vitamin K Antagonists] play a big role in the treatment of thromboembolic diseases, like a stroke or heart attack.<ref name="Goy">PMID:23034830</ref> [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common medication for this treatment, acting as a blood thinner. Warfarin binding in VKOR overall prevents the triggering of coagulation factors that form blood clots. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <ref name="Shen">PMID:33273012</ref> Shen, G., Cui, W., Cao, Q., Gao, M., Liu, H., Su, G., Gross, M. L., & Li, W. (2021). The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. ''The Journal of biological chemistry'', 296, 100145. https://doi.org/10.1074/jbc.RA120.015401 | ||
| - | + | ==References== | |
| - | + | <references/> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
Revision as of 18:06, 12 April 2022
| |||||||||||