We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Neurofibromin
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
Glutamine 61 of Ras is a residue that facilitates the conversion of GTP to GDP, turning Ras from its active state to inactive state. There is a catalytic water molecule that glutamine interacts with to position the molecule for a nucleophilic attack on the gamma phosphate of GTP. Mutations of this residue have been related to lower rates of hydrolysis. <ref name= ''Frech''>PMID:8136358</ref>. Tyrosine 32 makes water-mediated hydrogen bonds with the gamma phosphate of GTP. This position is also where Ras is phosphorylation to promote the activity of GTPase-activating proteins and GTP hydrolysis. <ref name= ''Bunda''>DOI:10.1038/ncomms9859</ref> | Glutamine 61 of Ras is a residue that facilitates the conversion of GTP to GDP, turning Ras from its active state to inactive state. There is a catalytic water molecule that glutamine interacts with to position the molecule for a nucleophilic attack on the gamma phosphate of GTP. Mutations of this residue have been related to lower rates of hydrolysis. <ref name= ''Frech''>PMID:8136358</ref>. Tyrosine 32 makes water-mediated hydrogen bonds with the gamma phosphate of GTP. This position is also where Ras is phosphorylation to promote the activity of GTPase-activating proteins and GTP hydrolysis. <ref name= ''Bunda''>DOI:10.1038/ncomms9859</ref> | ||
==RAS Complex== | ==RAS Complex== | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Downstream Effects=== | ===Downstream Effects=== | ||
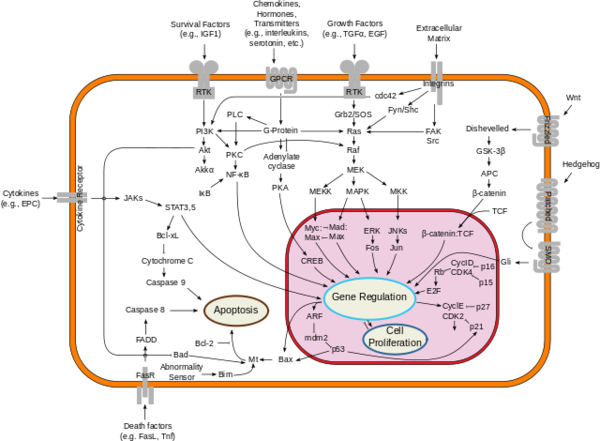
[[Image:Signal_transduction_pathways.png|600 px|right|thumb|Figure 2; By cybertory - This file was derived from: Signal transduction v1.png, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=12081090]] | [[Image:Signal_transduction_pathways.png|600 px|right|thumb|Figure 2; By cybertory - This file was derived from: Signal transduction v1.png, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=12081090]] | ||
Revision as of 19:20, 7 April 2022
| |||||||||||
References
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12138-42. Epub 2002 Sep 4. PMID:12213964 doi:http://dx.doi.org/10.1073/pnas.192453199
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994 Mar 22;33(11):3237-44. doi: 10.1021/bi00177a014. PMID:8136358 doi:http://dx.doi.org/10.1021/bi00177a014
- ↑ Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, Raught B, Zhang ZY, Zadeh G, Ohh M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015 Nov 30;6:8859. doi: 10.1038/ncomms9859. PMID:26617336 doi:http://dx.doi.org/10.1038/ncomms9859
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am. 2019 Nov;103(6):1035-1054. doi: 10.1016/j.mcna.2019.07.004. PMID:31582003 doi:http://dx.doi.org/10.1016/j.mcna.2019.07.004
Proteopedia Page Contributors and Editors (what is this?)
Jordyn K. Lenard, Ryan D. Adkins, Michal Harel, OCA, Jaime Prilusky