We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1705
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== General Structure == | == General Structure == | ||
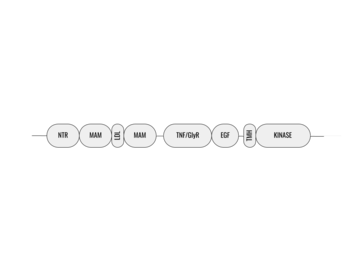
| - | Anaplastic lymphoma kinase is a <scene name='90/904310/Dimer/2'>homodimer</scene>, each monomer consisting of seven domains and two regions <ref name="Tongqing">PMID:34819665</ref>. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), <scene name='90/904310/Tnf_highlighted_monomer/2'>tumor necrosis factor receptor-like domain</scene> (TNF), <scene name='90/904310/Glyr_highlighted_monomer/1'>glycine rich region</scene>(GlyR), <scene name='90/904310/Egf_highlighted_monomer/1'>epidermal growth factor receptor-like domain</scene> (EGF), transmembrane α-helix (TMH), kinase domain <ref name="Reshetnyak">PMID:34819673</ref>. The structures of the N-terminal region, MAM, and LDL have not been determined. The glycine rich region is a part of the TNF domain. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular region. | + | Anaplastic lymphoma kinase is a <scene name='90/904310/Dimer/2'>homodimer</scene>, each monomer consisting of seven domains and two regions <ref name="Tongqing">PMID:34819665</ref>. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), <scene name='90/904310/Tnf_highlighted_monomer/2'>tumor necrosis factor receptor-like domain</scene> (TNF), <scene name='90/904310/Glyr_highlighted_monomer/1'>glycine rich region</scene>(GlyR), <scene name='90/904310/Egf_highlighted_monomer/1'>epidermal growth factor receptor-like domain</scene> (EGF), transmembrane α-helix (TMH), kinase domain <ref name="Reshetnyak">PMID:34819673</ref>. The NTR functions as a signal peptide, the structure of which is yet to be determined. Though the biological roles and structures of MAM and LDL have not been determined, they are a very unique component to ALK. ALK is the only RTK that has two MAM domains and a LDL domain. Studies of other MAM domains have suggested that MAM may play a role in cell-cell interactions through homophilic binding <ref name="Huang">PMID: 30400214</ref>. The structures of the N-terminal region, MAM, and LDL have not been determined. The glycine rich region is a part of the TNF domain. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular region. |
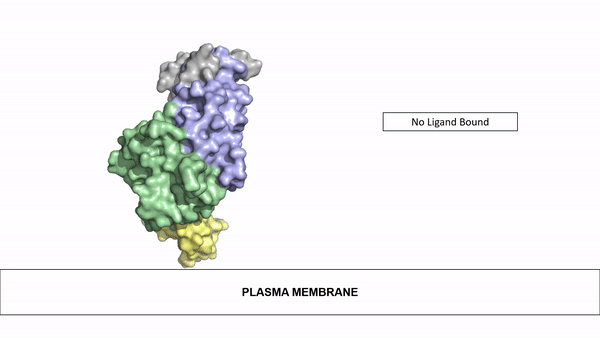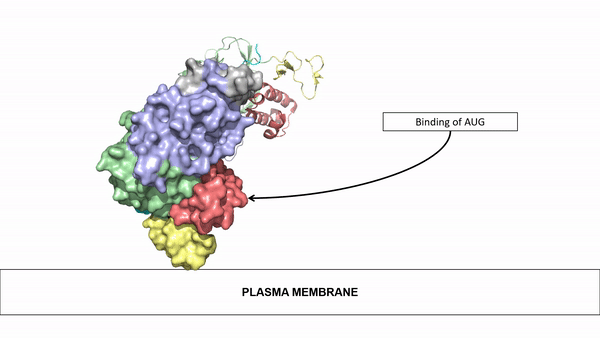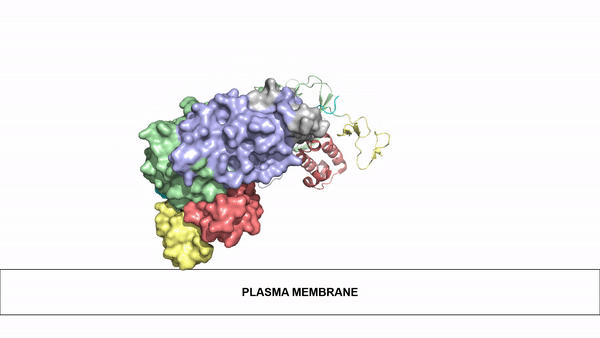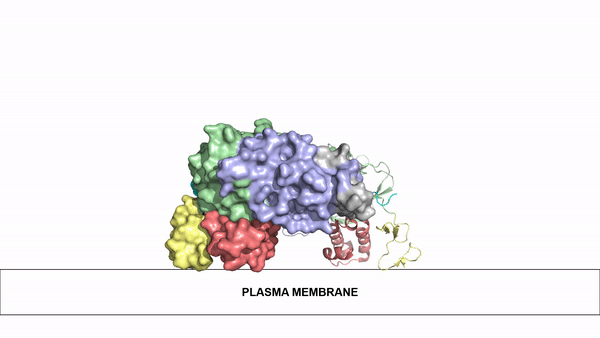
=== Ligand Binding=== | === Ligand Binding=== | ||
Revision as of 13:40, 12 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Lewis RT, Bode CM, Choquette D, Potashman M, Romero K, Stellwagen JC, Teffera Y, Moore E, Whittington DA, Chen H, Epstein LF, Emkey R, Andrews PS, Yu V, Saffran DC, Xu M, Drew AE, Merkel P, Szilvassy S, Brake RL. The discovery and optimization of a novel class of potent, selective and orally bioavailable Anaplastic Lymphoma Kinase (ALK) Inhibitors with potential utility for the treatment of cancer. J Med Chem. 2012 Jun 26. PMID:22734674 doi:10.1021/jm3005866
- ↑ 6.0 6.1 Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013 Apr;2(2):91-7. doi: 10.4103/2278-330X.110506. PMID:24455567 doi:http://dx.doi.org/10.4103/2278-330X.110506
- ↑ 7.0 7.1 Wang Q, Zorn JA, Kuriyan J. A structural atlas of kinases inhibited by clinically approved drugs. Methods Enzymol. 2014;548:23-67. doi: 10.1016/B978-0-12-397918-6.00002-1. PMID:25399641 doi:http://dx.doi.org/10.1016/B978-0-12-397918-6.00002-1
Student Contributors
- Kaylin Todor
- Rebekah White