We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
VEGF signaling pathway
From Proteopedia
(Difference between revisions)
| Line 47: | Line 47: | ||
See also [[Kinase-linked, enzyme-linked and related receptors]]. | See also [[Kinase-linked, enzyme-linked and related receptors]]. | ||
| - | ==Biological Function == | + | ===Biological Function === |
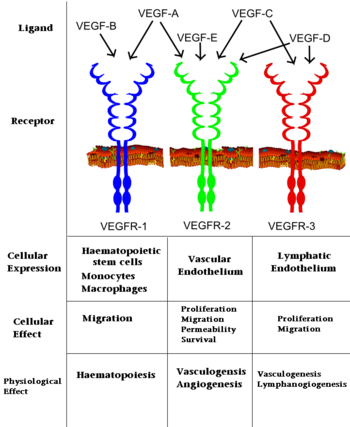
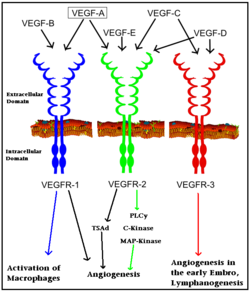
The VEGFRs are a family of tyrosine kinase receptors on the surface of different cells depending on family identity. VEGFR-1 is expressed on haematopoietic stem cells, monocytes, and vascular endothelial cells. VEGFR-2 is expressed on vascular endothelial cells and lymphatic endothelial cells, while VEGFR-3 is only expressed on lymphatic endothelial cells<ref>PMID:16633338</ref>. | The VEGFRs are a family of tyrosine kinase receptors on the surface of different cells depending on family identity. VEGFR-1 is expressed on haematopoietic stem cells, monocytes, and vascular endothelial cells. VEGFR-2 is expressed on vascular endothelial cells and lymphatic endothelial cells, while VEGFR-3 is only expressed on lymphatic endothelial cells<ref>PMID:16633338</ref>. | ||
| Line 53: | Line 53: | ||
In terms of function, VEGFR-1 is required for the recruitment of haematopoietic stem cells as well as the migration of monocytes and macrophages while VEGFR-2 regulates vascular endothelial function and VEGFR-3 regulates lymphatic endothelial cell function.<ref>PMID: 17658244</ref> VEGFR-2 has been the focus of the most research as it is the major signal transducer of both physioligcal, and perhaps more importantly, pathological angiogenesis, especially in cancerous tumors. VEGFR-2 is of critical importance to the body as exemplified by Shalaby ''et al.'' who demonstrated that VEGFR-2 gene knockout mice die at E8-8.5 due to lack of vasculogenesis.<ref>PMID:7596453</ref> The signal cascade initiated by binding VEGF to VEGFR is dependent upon specific sites of phosphorylation in the VEGFR structure and the interaction between these phosphorylated sites and other signaling molecules. | In terms of function, VEGFR-1 is required for the recruitment of haematopoietic stem cells as well as the migration of monocytes and macrophages while VEGFR-2 regulates vascular endothelial function and VEGFR-3 regulates lymphatic endothelial cell function.<ref>PMID: 17658244</ref> VEGFR-2 has been the focus of the most research as it is the major signal transducer of both physioligcal, and perhaps more importantly, pathological angiogenesis, especially in cancerous tumors. VEGFR-2 is of critical importance to the body as exemplified by Shalaby ''et al.'' who demonstrated that VEGFR-2 gene knockout mice die at E8-8.5 due to lack of vasculogenesis.<ref>PMID:7596453</ref> The signal cascade initiated by binding VEGF to VEGFR is dependent upon specific sites of phosphorylation in the VEGFR structure and the interaction between these phosphorylated sites and other signaling molecules. | ||
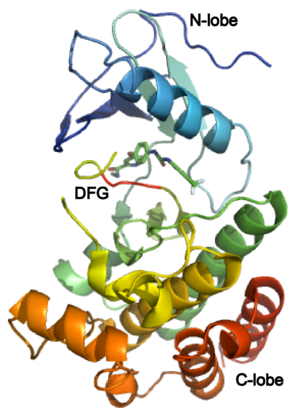
| - | ==Structure of VEGFR-2 and Biology== | + | ===Structure of VEGFR-2 and Biology=== |
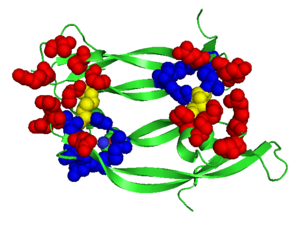
The structure of VEGFR-2 can been seen at the right. VEGF-A binds to the second and third extracellular Ig-like domains of VEGFR-2 with a 10-fold lower affinity than it does to the second Ig-like domain of VEGFR-1, despite the fact that VEGFR-2 is the principal mediator of several physiological effects on endothelial cells including proliferation, migration, and survival.<ref> PMID:9813036</ref> Binding of VEGF to the domains 2 and 3 of a VEGFR-2 monomer increases the probability that an additional VEGFR-2 binds the tethered ligand to form a dimmer. Once the two receptors are cross-linked, interactions between their membrane-proximal domain 7s stabilize the dimmer significantly. This dimerization and stabilization allows for precise positioning of the intracellular kinase domains, resulting in autophosphorylation and subsequent activation of the classical extracellular signal-regulated kinases (ERK) pathway.<ref>PMID:17293873</ref>. | The structure of VEGFR-2 can been seen at the right. VEGF-A binds to the second and third extracellular Ig-like domains of VEGFR-2 with a 10-fold lower affinity than it does to the second Ig-like domain of VEGFR-1, despite the fact that VEGFR-2 is the principal mediator of several physiological effects on endothelial cells including proliferation, migration, and survival.<ref> PMID:9813036</ref> Binding of VEGF to the domains 2 and 3 of a VEGFR-2 monomer increases the probability that an additional VEGFR-2 binds the tethered ligand to form a dimmer. Once the two receptors are cross-linked, interactions between their membrane-proximal domain 7s stabilize the dimmer significantly. This dimerization and stabilization allows for precise positioning of the intracellular kinase domains, resulting in autophosphorylation and subsequent activation of the classical extracellular signal-regulated kinases (ERK) pathway.<ref>PMID:17293873</ref>. | ||
The tyrosine kinase domain of VEGFR-2 is separated into two segments with a 70 amino acid long kinase insert region. Upon binding VEGFA and subsequent dimerization, VEGFR-2 is autophosphoryalted at the carboxy terminal tail and kinase insert region. Six tyrosine residues of VEGFR2 are autophosphorylated (see Fig.1<ref>PMID:15962004</ref>). <scene name='41/411436/Cv/2'>Auto-phosphorylation of residues1054 and 1059</scene> within the activation loop of VEGFR2 leads to increased kinase activity<ref>PMID:10037737</ref>.<br /> | The tyrosine kinase domain of VEGFR-2 is separated into two segments with a 70 amino acid long kinase insert region. Upon binding VEGFA and subsequent dimerization, VEGFR-2 is autophosphoryalted at the carboxy terminal tail and kinase insert region. Six tyrosine residues of VEGFR2 are autophosphorylated (see Fig.1<ref>PMID:15962004</ref>). <scene name='41/411436/Cv/2'>Auto-phosphorylation of residues1054 and 1059</scene> within the activation loop of VEGFR2 leads to increased kinase activity<ref>PMID:10037737</ref>.<br /> | ||
| - | ==Medical significance== | + | ===Medical significance=== |
[[Image: Sorafenib.png|300px|left|thumb| [[Sorafenib]], anti VEGFR drug targeting the MAP Kinase pathway, marketed by Bayer for Renal and Liver [[Cancer]].]] | [[Image: Sorafenib.png|300px|left|thumb| [[Sorafenib]], anti VEGFR drug targeting the MAP Kinase pathway, marketed by Bayer for Renal and Liver [[Cancer]].]] | ||
{{Clear}} | {{Clear}} | ||
Revision as of 14:04, 13 April 2022
| |||||||||||
References
- ↑ Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983-5. PMID:6823562
- ↑ Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851-8. PMID:2735925
- ↑ Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581-611. PMID:15294883 doi:10.1210/er.2003-0027
- ↑ Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005 Jan 21;280(3):2126-31. Epub 2004 Nov 12. PMID:15542594 doi:10.1074/jbc.M411395200
- ↑ Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843-5. PMID:1279431 doi:http://dx.doi.org/10.1038/359843a0
- ↑ Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4-25. PMID:9034784
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001 Mar;114(Pt 5):853-65. PMID:11181169
- ↑ Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7192-7. PMID:9207067
- ↑ Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996 Mar 8;271(10):5638-46. PMID:8621427
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Oefner C, D'Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921-6. PMID:1396586
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. Identification of placenta growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem. 2004 Oct 15;279(42):43929-39. Epub 2004 Jul 21. PMID:15272021 doi:10.1074/jbc.M401418200
- ↑ Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004 Oct;30(5):427-46. PMID:15488020 doi:10.1111/j.1365-2990.2004.00600.x
- ↑ Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol. 2010 Jun 17. PMID:20662002 doi:10.1002/path.2748
- ↑ http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/
- ↑ Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785-8. PMID:22130231
- ↑ Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. PMID:16633338 doi:10.1038/nrm1911
- ↑ Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007 Oct;19(10):2003-12. Epub 2007 Jun 12. PMID:17658244 doi:10.1016/j.cellsig.2007.05.013
- ↑ Gallina P, Nohra G, Cioloca C, Meder JF, Roux FX. [Multiple cavernoma of delayed appearance] Neurochirurgie. 1994;40(5):322-5. PMID:7596453
- ↑ Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998 Nov 20;273(47):31283-8. PMID:9813036
- ↑ Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007 Mar;14(3):249-50. Epub 2007 Feb 11. PMID:17293873 doi:10.1038/nsmb1202
- ↑ Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005 Jul 6;24(13):2342-53. Epub 2005 Jun 16. PMID:15962004 doi:10.1038/sj.emboj.7600709
- ↑ Kendall RL, Rutledge RZ, Mao X, Tebben AJ, Hungate RW, Thomas KA. Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J Biol Chem. 1999 Mar 5;274(10):6453-60. PMID:10037737
- ↑ Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002 Oct;2(10):795-803. PMID:12360282 doi:10.1038/nrc909
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
- ↑ Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001 Sep;7(9):987-9. PMID:11533692 doi:10.1038/nm0901-987