Sandbox Reserved 1709
From Proteopedia
| Line 4: | Line 4: | ||
== Introduction== | == Introduction== | ||
=== Biological Role === | === Biological Role === | ||
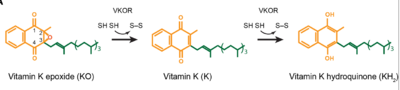
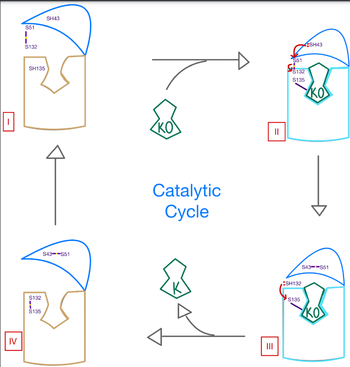
| - | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein <ref>DOI 10.1126</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] Vitamin K hydroquinone (KH2) (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into Vitamin K. Then, a second pair of cysteine residues will reduce Vitamin K | + | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein <ref>DOI 10.1126</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] Vitamin K hydroquinone (KH2) (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into Vitamin K. Then, a second pair of cysteine residues will reduce Vitamin K int2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade. |
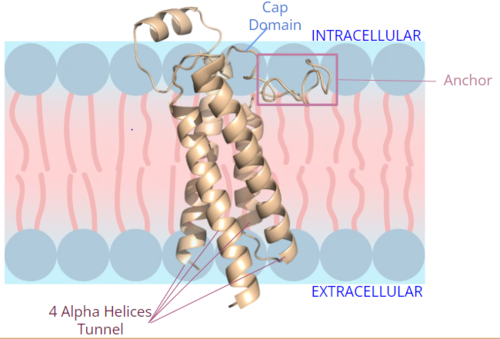
| - | + | [[Image:VKOR_mechanism_2D.png|400 px|right|thumo the final product, KH2 (Figure 1). One of VKORs primary roles is to assist in blood coagulation through this KH2 regeneration mechanism. With Vitamin K as a cofactor, the [https://www.britannica.com/science/bleeding/The-extrinsic-pathway-of-blood-coagulation#ref64617 γ-carboxylase] enzyme will enact post-translational modification on KH2, oxidizing it back to KO. The oxidation of KHb|Figure 2. Orientation and interactions of cap domain, anchor domain, and helical tunnel within the cell membrane.]] | |
=== Author's Notes === | === Author's Notes === | ||
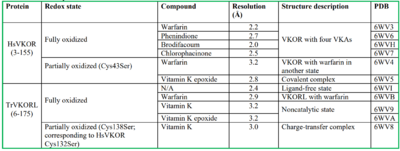
Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. Crystal structures of VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA)<ref>DOI 10.1126</ref>. VKA substrates utilized were anticoagulants, namely [https://en.wikipedia.org/wiki/Warfarin Warfarin], [https://en.wikipedia.org/wiki/Brodifacoum Brodifacoum], [https://en.wikipedia.org/wiki/Phenindione Phenindione], and [https://en.wikipedia.org/wiki/Chlorophacinone Chlorophacinone]. Second, VKOR-like homologs were utilized to aid in structure classification. Homologs refer to specific cysteine residues that have been mutated to serine to facilitate capturing a stable conformation state. Homologs were mainly isolated from human VKOR with some isolated from the pufferfish ''Takifugu rubripes''. Furthermore, all of the structures used have been processed to remove a beta barrel at the south end of VKOR that served no purpose in function of the enzyme. This also allowed for the residue numbering to be reassigned and more closely replicate the human VKOR. | Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. Crystal structures of VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA)<ref>DOI 10.1126</ref>. VKA substrates utilized were anticoagulants, namely [https://en.wikipedia.org/wiki/Warfarin Warfarin], [https://en.wikipedia.org/wiki/Brodifacoum Brodifacoum], [https://en.wikipedia.org/wiki/Phenindione Phenindione], and [https://en.wikipedia.org/wiki/Chlorophacinone Chlorophacinone]. Second, VKOR-like homologs were utilized to aid in structure classification. Homologs refer to specific cysteine residues that have been mutated to serine to facilitate capturing a stable conformation state. Homologs were mainly isolated from human VKOR with some isolated from the pufferfish ''Takifugu rubripes''. Furthermore, all of the structures used have been processed to remove a beta barrel at the south end of VKOR that served no purpose in function of the enzyme. This also allowed for the residue numbering to be reassigned and more closely replicate the human VKOR. | ||
| Line 17: | Line 17: | ||
=== Cap Domain === | === Cap Domain === | ||
| - | [[Image:VKOR_in_cell_membrane.png|500 px|right|thumb|Figure 2. Orientation and interactions of cap domain, anchor domain, and helical tunnel within the cell membrane.]] | + | [[Image:VKOR_in_cell_membrane.png|500 px|right|thumb|Figure 2. Orientation and interactions of the VKOR components cap domain, anchor domain, and helical tunnel within the cell membrane.]] |
A key part of VKOR is the function of the <scene name='90/904314/Cap_domain/4'>cap domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide bridge</scene> between C43 and C51, and polar interactions from D44. These interactions are broken up by reactive cysteines to induce different conformations and help facilitate this transition from the open conformation to the closed conformation during the activation of Vitamin K. | A key part of VKOR is the function of the <scene name='90/904314/Cap_domain/4'>cap domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide bridge</scene> between C43 and C51, and polar interactions from D44. These interactions are broken up by reactive cysteines to induce different conformations and help facilitate this transition from the open conformation to the closed conformation during the activation of Vitamin K. | ||
Revision as of 12:06, 14 April 2022
Vitamin K Epoxide Reductase
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]