We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1725
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
==Protein Structure== | ==Protein Structure== | ||
=== Structural Overview === | === Structural Overview === | ||
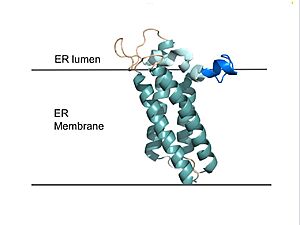
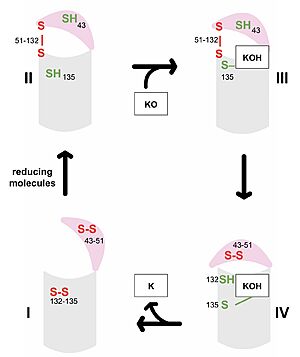
| - | VKOR consists of four <scene name='90/904330/Transmembranehelices1/5'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane (Figure 2). Helices one and two are <scene name='90/904330/Betahairpin2/ | + | VKOR consists of four <scene name='90/904330/Transmembranehelices1/5'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane (Figure 2). Helices one and two are <scene name='90/904330/Betahairpin2/4'>connected</scene> by <b><span class="text-brown">Loop 1</span></b> and the <b><span class="text-orange">beta hairpin</span></b> region which contains two of the active cysteines, Cys43 and Cys51; these cysteines, along with Cys132 and Cys135, are essential for reduction and structural changes discussed in the next section<ref name="Liu">PMID:33154105</ref>. VKOR also has a <scene name='90/904330/Capdomain/3'>cap domain</scene> consisting of a <b><span class="text-blue">helix</span></b>, <b><span class="text-lightmagenta">loop</span></b>, and <b><span class="text-olive">anchor</span></b>. The <b><span class="text-olive">anchor</span></b> stabilizes the cap domain by attaching it to the surface of the ER membrane<ref name="Liu">PMID:33154105</ref>. The <b><span class="text-lightmagenta">loop</span></b> helps stabilize one of the substrate-binding amino acids, Asn80<ref name="Liu">PMID:33154105</ref>. The <b><span class="text-blue">helix</span></b> is involved in stabilization of certain disulfide bonds and structural changes as part of the catalytic cycle discussed below<ref name="Liu">PMID:33154105</ref>. |
=== Author's Note === | === Author's Note === | ||
Revision as of 19:49, 18 April 2022
–
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
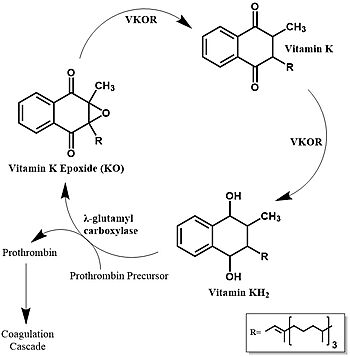
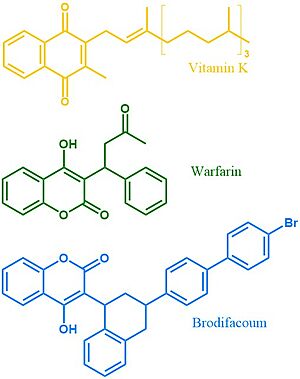
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ 1.0 1.1 Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 Blanchard RA, Furie BC, Jorgensen M, Kruger SF, Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med. 1981 Jul 30;305(5):242-8. doi: 10.1056/NEJM198107303050502. PMID:6165889 doi:http://dx.doi.org/10.1056/NEJM198107303050502
- ↑ Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982 Nov 9;21(23):6011-8. doi: 10.1021/bi00266a044. PMID:6758841 doi:http://dx.doi.org/10.1021/bi00266a044
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ 5.0 5.1 Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018 Aug 9;132(6):647-657. doi: 10.1182/blood-2018-01-830901. Epub 2018, May 9. PMID:29743176 doi:http://dx.doi.org/10.1182/blood-2018-01-830901
- ↑ 7.0 7.1 Chong YK, Mak TW. Superwarfarin (Long-Acting Anticoagulant Rodenticides) Poisoning: from Pathophysiology to Laboratory-Guided Clinical Management. Clin Biochem Rev. 2019 Nov;40(4):175-185. doi: 10.33176/AACB-19-00029. PMID:31857739 doi:http://dx.doi.org/10.33176/AACB-19-00029
Student Contributors
Izabella Jordan, Emma Varness