Introduction
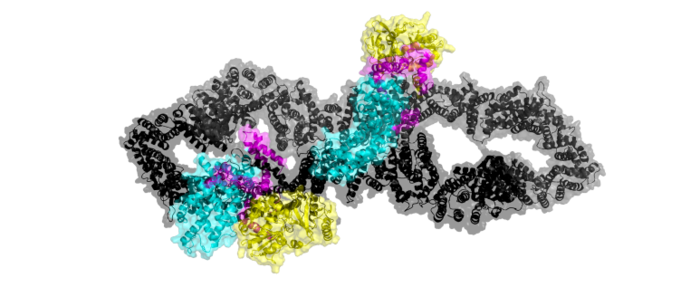
Neurofibromatosis Type 1 is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the GTPase-activating protein neurofibromin. Neurofibromin is closely involved in signaling pathways such as MAPK/ERK, P13K/AKT/mTOR, and other cell signaling pathways that use Ras [1]. Decreased activity of neurofibromin due to mutation can lead to tumor growth along nerves. As it is ubiquitous expression, NF1 misregulation can cause systemic tumor growth. Neurofibromin is localized to the cytosol but is recruited to the plasma membrane to inactivate Ras. The structure of neurofibromin was determined by high-resolution single particle cryo-EM. These structures illustrated the domain architecture and conformational changes in neurofibromin, controlling Ras binding and inactivation. [1][2][3][4]
Function
Neurofibromin is a
GTPase-activating protein that binds to
Ras, a
GTPase, to increase its inherent hydrolysis of GTP to GDP (Figure 1). This inactivates the cell signaling of Ras until reactivated by
GTP exchange. Neurofibromin has two structural conformations, the open and closed conformation. Neurofibromin only binds to Ras in its open conformation.
[2][3][4] 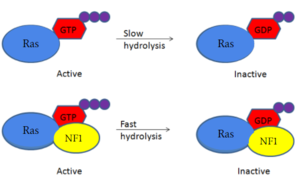
Figure 1: Shifted rate of of Ras GTP hydrolysis when bound to neurofibromin. The speed of GTP hydrolysis is significantly increased when bound to neurofibromin. Ras is inactive when bound to GDP and active when bound to GTP
Structure
Neurofibromin is a protein dimer that exists in and conformations. The exact initiation of structural rearrangement is currently unknown and a point of further research. DOI:10.1038/39470</ref>[3]. Both protomers contain 5 separate domains, a N-HEAT ARM, C-HEAT ARM, GRD active site, GAPex subdomain, and Sec14-PH domain.
Domains
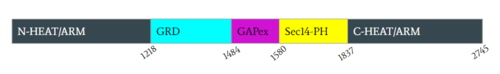
Figure 2: Domains for NF1 and the pertinent colors. Numerical values show domain locations along the polypeptide chain.
N-C HEAT ARM
The first domain are two separate but very similar domains; the N-HEAT ARM(N-Terminal) and C-HEAT ARM(C-Terminal). HEAT and ARM are acronyms for a repeating structure of helices. Structurally, the N-HEAT ARMs consist of various helices and loops that interconnect on one of the existing protomers. The N-HEAT ARM is critical in stabilization and linking the catalytic and core domains. The N-HEAT ARM in its linkage with the GRD catalytic domain also has a direct impact on the relative conformational change from the active (Ras BOUND) to inactive (Ras unbound) states. The Zn2+ binding site also extends from the N-HEAT ARM just before the GRD and SEC14-PH domains. The C-HEAT Arm plays similar roles to the N-HEAT ARM by stabilizing and linking the GRD and SEC14-PH domain and contributing to the conformational changes from the closed to open state.[3][4]
GRD
Neurofibromin’s main catalytic domain is the GRD active site. Linked structurally to both HEAT ARM’s, it consists of mainly loops and helices. There is one single GRD binding site per protomer. In the closed state, Ras cannot bind the GRD site due to a steric hindrance in which Ras clashes with the N-HEAT ARM. Within the GRD site, the critical active site residue is Arg1276.[3][4]
GAPex
The GAPex subdomain of the GRD site lies between the Sec14-PH and GRD catalytic sites. This domain is non-catalytic and structurally consists of various loops and helices. Its main function is to bind SPRED-1. SPRED-1 is a recruiter protein that binds to this subdomain in the cytosol and recruits Neurofibromin to the plasma membrane. [3][4]
Sec14-PH
The Sec14-PH domain is linked and extends out from the HEAT ARM’s. Sec14-PH functions as a membrane associated domain and using a hydrophobic cavity binds to neurofibromin the plasma membrane. The also blocks Sec14-PH binding to the cell membrane due to blocking by GRD. [3][4]
Conformations
Closed conformation
Ras is unable to bind to the GRD active site when both of the neurofibromin protomers are in the closed conformation. In the , one protomer has its domains shifted by a 130° rotation of three separate conformational change linkers. That rotation places in an orientation that in the GRD site (Figure 3). Ras binding to the GRD site is inhibited by steric occlusion from the N-HEAT ARM. The can exist naturally without any form of stabilization but exists in a natural equilibrium with the .[2] [3][4]
Zinc Stabilized
The is stabilized by a zinc ion that prevents the shift back to the . Zinc binding is chelated by three residues (. These three residues are contributed by the N-HEAT domain (Cys1032) and the GAPex-subdomain (His1558 and His1576). Zinc stabilization keeps Neurofibromin in the , inhibiting Ras binding.[2][3][4]
Open conformation
In the open state, one protomer is shifted to allow Ras binding, while the other protomer remain in the closed conformation.
In the one protomer is rotated 90°, facilitating binding between RAS and the
in the GRD site. To allow Ras binding, the GRD and Sec14-PH domains are reoriented away from one another and the GRD site is accessible for Ras binding. This conformational change to the open conformation is driven by rearrangement of three separate linkers (L1, L2, L3).[2] [3][4]
Conformational Change Linkers
These three helical linkers (L1, L2, and L3)(
and the
) undergo rotations to relocate the GRD and Sec14-PH sites. Linker 1 (L1) consists of a loop connected by two helices from L1173-M1215 and is the main contributor in rotation of the GRD domain. The rotation of L1 to the causes N-HEAT ARM α helix 48 and GRD helix 49 to extend out, aligning to form a hinge point at G1190. The GRD relocation is assisted by Sec14-PH relocation, which is initiated by Linker 3(L3) from Q1835 to G1852. Movement of L3 is further supported by rearrangement of the proline rich section of the C-HEAT ARM. L1 and L3 also move closer to each other in the initiating rearrangement of the GRD and Sec14-PH domain. Linker 2 (L2) consists of residues G1547-T1565 and begins at helix 63, the final helix of the GRD site, and connects into the short loop of α helix 65 of the Sec14-PH domain and also assists in shifting the Sec14-PH away from the GRD site. The combination of these three linkers are largely responsible for the conformational shift of the
and
.[3][4]
Ras Binding
Arg1276 is the critical residue within the GRD site for Ras activation. In the open conformation, the arginine finger binds to the backbone γ-carbon of Y32 in Ras to assist in eventual hydrolysis of GTP. In the closed conformation, this interaction between Y32 and GTP is blocked by E31. Removal of the inhibition of E31 from Ras by R1276 from Neurofibromin allows for the normal function of neurofibromin in the rapid rate increase of GTP hydrolysis upon Ras binding. Mutations to the arginine finger slow GTPase activating reaction of neurofibromin.[2][3][4]
SPRED 1
SPRED 1 is another peripheral protein that interacts with the GRD domain of Neurofibromin. SPRED 1 recruits Neurofibromin from the cytosol to the plasma membrane to interact with Ras. Binding of SPRED 1 can occur in either the open or closed conformation and causes a structural rearrangement of the GRD domain and GAPex subdomain that has yet to be structuralized.[3][4]
Clinical Relevance
Germline mutations are common in NF1 and often cause genetic tumor syndrome through misregulation of the Ras signaling pathway. Somatic mutations among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, tumors develop along the deep epidermis layer of the skin. Understanding how mutations affect the structure and function of neurofibromin will allow for advancements in treatment.
DOI:10.3390/cells9112365</ref>[5][6]
FINAL SCENES COPY AND PASTE
Open/Closed Conformation Scenes
Open-
Closed-
Domain Scenes-
Linkers Open/Closed-
Closed Linker-
Button for closed zoomed in linkers"
Open Linker-
Button for open zoomed in linkers"
Zinc Binding Site Fixed-
RAS Bound in Open Fixed-
Button for open zoomed in Arg 1276"
Open Zoomed out Molecular Interactions:
Button for open zoomed in Arg Molecular Interactions
RAS Bound in Closed Fixed-
Button for closed zoomed in Arg 1276"
Molecular Interactions closed R1276, E31, Y32:
Button for molecular interactions closed R1276, E31, Y32
Spred1-
References placeholders
This section will be removed it is just for copy and paste purposes. We will add the PDB code names as references in the captions of each scene.
[1]
[2]
[5]
[3]
[4]
[6]
References
- ↑ 1.0 1.1 1.2 Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Bourne HR. G proteins. The arginine finger strikes again. Nature. 1997 Oct 16;389(6652):673-4. doi: 10.1038/39470. PMID:9338774 doi:http://dx.doi.org/10.1038/39470
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ 5.0 5.1 Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest. 2017 Feb;97(2):146-157. doi: 10.1038/labinvest.2016.142. Epub 2017, Jan 9. PMID:28067895 doi:http://dx.doi.org/10.1038/labinvest.2016.142
- ↑ 6.0 6.1 Sabatini C, Milani D, Menni F, Tadini G, Esposito S. Treatment of neurofibromatosis type 1. Curr Treat Options Neurol. 2015 Jun;17(6):355. doi: 10.1007/s11940-015-0355-4. PMID:25917340 doi:http://dx.doi.org/10.1007/s11940-015-0355-4