We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1706
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==Introduction== | ==Introduction== | ||
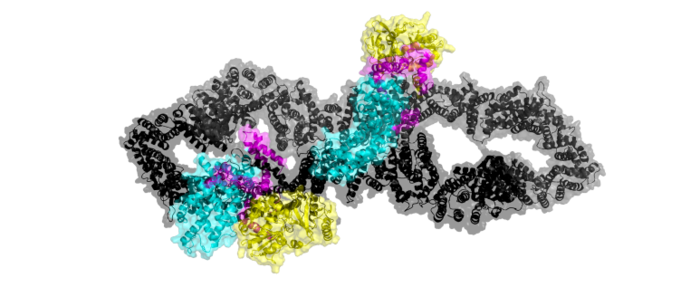
[https://en.wikipedia.org/wiki/Neurofibromatosis_type_I Neurofibromatosis Type 1] is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] neurofibromin. Neurofibromin is closely involved in signaling pathways such as [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK/ERK], [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway P13K/AKT/mTOR], and other cell signaling pathways that use [https://en.wikipedia.org/wiki/Ras_GTPase Ras] <ref name="Bergoug"> DOI:10.3390/cells9112365</ref>. Decreased activity of neurofibromin due to mutation can lead to tumor growth along nerves. As it is ubiquitous expression, NF1 misregulation can cause systemic tumor growth. Neurofibromin is localized to the [https://en.wikipedia.org/wiki/Cytosol cytosol] but is recruited to the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane] to inactivate [https://en.wikipedia.org/wiki/Ras_GTPase Ras]. The structure of neurofibromin was determined by high-resolution single particle [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-EM]. These structures illustrated the domain architecture and conformational changes in neurofibromin, controlling Ras binding and inactivation. <ref name="Bergoug"> DOI:10.3390/cells9112365</ref><ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | [https://en.wikipedia.org/wiki/Neurofibromatosis_type_I Neurofibromatosis Type 1] is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] neurofibromin. Neurofibromin is closely involved in signaling pathways such as [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK/ERK], [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway P13K/AKT/mTOR], and other cell signaling pathways that use [https://en.wikipedia.org/wiki/Ras_GTPase Ras] <ref name="Bergoug"> DOI:10.3390/cells9112365</ref>. Decreased activity of neurofibromin due to mutation can lead to tumor growth along nerves. As it is ubiquitous expression, NF1 misregulation can cause systemic tumor growth. Neurofibromin is localized to the [https://en.wikipedia.org/wiki/Cytosol cytosol] but is recruited to the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane] to inactivate [https://en.wikipedia.org/wiki/Ras_GTPase Ras]. The structure of neurofibromin was determined by high-resolution single particle [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-EM]. These structures illustrated the domain architecture and conformational changes in neurofibromin, controlling Ras binding and inactivation. <ref name="Bergoug"> DOI:10.3390/cells9112365</ref><ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
==Function== | ==Function== | ||
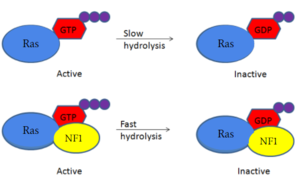
Neurofibromin is a [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] that binds to [https://en.wikipedia.org/wiki/Ras_GTPase Ras], a [https://en.wikipedia.org/wiki/GTPase GTPase], to increase its inherent hydrolysis of GTP to GDP (Figure 1). This inactivates the cell signaling of Ras until reactivated by [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP] exchange. Neurofibromin has two structural conformations, the open and closed conformation. Neurofibromin only binds to Ras in its open conformation. <ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> [[Image:RasNeurofibrominMech.PNG|300px|right|thumb|Figure 1: Shifted rate of of Ras GTP hydrolysis when bound to neurofibromin. The speed of GTP hydrolysis is significantly increased when bound to neurofibromin. Ras is inactive when bound to GDP and active when bound to GTP ]] | Neurofibromin is a [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] that binds to [https://en.wikipedia.org/wiki/Ras_GTPase Ras], a [https://en.wikipedia.org/wiki/GTPase GTPase], to increase its inherent hydrolysis of GTP to GDP (Figure 1). This inactivates the cell signaling of Ras until reactivated by [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP] exchange. Neurofibromin has two structural conformations, the open and closed conformation. Neurofibromin only binds to Ras in its open conformation. <ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> [[Image:RasNeurofibrominMech.PNG|300px|right|thumb|Figure 1: Shifted rate of of Ras GTP hydrolysis when bound to neurofibromin. The speed of GTP hydrolysis is significantly increased when bound to neurofibromin. Ras is inactive when bound to GDP and active when bound to GTP ]] | ||
| + | |||
==Structure== | ==Structure== | ||
Neurofibromin is a [https://en.wikipedia.org/wiki/Protein_dimer protein dimer] that exists in <scene name='90/904311/Closed_conformation/8'>closed</scene> and <scene name='90/904311/Open_conformation/14'> open</scene> conformations. The exact initiation of structural rearrangement is currently unknown and a point of further research. DOI:10.1038/39470</ref><ref name="Lupton"> <ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref>. Both protomers contain 5 separate domains, a N-HEAT ARM, C-HEAT ARM, GRD active site, GAPex subdomain, and Sec14-PH domain. | Neurofibromin is a [https://en.wikipedia.org/wiki/Protein_dimer protein dimer] that exists in <scene name='90/904311/Closed_conformation/8'>closed</scene> and <scene name='90/904311/Open_conformation/14'> open</scene> conformations. The exact initiation of structural rearrangement is currently unknown and a point of further research. DOI:10.1038/39470</ref><ref name="Lupton"> <ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref>. Both protomers contain 5 separate domains, a N-HEAT ARM, C-HEAT ARM, GRD active site, GAPex subdomain, and Sec14-PH domain. | ||
| + | |||
===Domains=== | ===Domains=== | ||
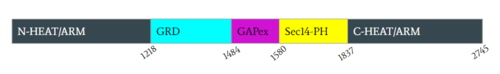
[[Image:Domain.PNG|500px|right|thumb|Figure 2: Domains for NF1 with their location on the <scene name='90/904311/Domainintro_open/11'>neurofibromin monomer</scene> and the pertinent colors. Numerical values show domain locations along the polypeptide chain.]] | [[Image:Domain.PNG|500px|right|thumb|Figure 2: Domains for NF1 with their location on the <scene name='90/904311/Domainintro_open/11'>neurofibromin monomer</scene> and the pertinent colors. Numerical values show domain locations along the polypeptide chain.]] | ||
| + | |||
====N-C HEAT ARM==== | ====N-C HEAT ARM==== | ||
The first domain are two separate but very similar domains; <scene name='90/904311/N-heatarm_open/2'>N-HEAT ARM</scene>(N-Terminal) and <scene name='90/904311/C-heatarm_open/2'>C-HEAT ARM</scene>(C-Terminal). [https://en.wikipedia.org/wiki/HEAT_repeat HEAT] and [https://en.wikipedia.org/wiki/Armadillo_repeat ARM] are acronyms for a trope of repeating helical structures. Structurally, the N-HEAT ARMs consist of various helices and loops that interconnect on one of the existing protomers. The N-HEAT ARM is critical in stabilization and linking the catalytic and core domains. The N-HEAT ARM in its linkage with the GRD catalytic domain also has a direct impact on the relative conformational change from the active (Ras BOUND) to inactive (Ras unbound) states. The Zn2+ binding site also extends from the N-HEAT ARM just before the GRD and SEC14-PH domains. The C-HEAT Arm plays similar roles to the N-HEAT ARM by stabilizing and linking the GRD and SEC14-PH domain and contributing to the conformational changes from the closed to open state.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | The first domain are two separate but very similar domains; <scene name='90/904311/N-heatarm_open/2'>N-HEAT ARM</scene>(N-Terminal) and <scene name='90/904311/C-heatarm_open/2'>C-HEAT ARM</scene>(C-Terminal). [https://en.wikipedia.org/wiki/HEAT_repeat HEAT] and [https://en.wikipedia.org/wiki/Armadillo_repeat ARM] are acronyms for a trope of repeating helical structures. Structurally, the N-HEAT ARMs consist of various helices and loops that interconnect on one of the existing protomers. The N-HEAT ARM is critical in stabilization and linking the catalytic and core domains. The N-HEAT ARM in its linkage with the GRD catalytic domain also has a direct impact on the relative conformational change from the active (Ras BOUND) to inactive (Ras unbound) states. The Zn2+ binding site also extends from the N-HEAT ARM just before the GRD and SEC14-PH domains. The C-HEAT Arm plays similar roles to the N-HEAT ARM by stabilizing and linking the GRD and SEC14-PH domain and contributing to the conformational changes from the closed to open state.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
====GRD==== | ====GRD==== | ||
Neurofibromin’s main catalytic domain is the <scene name='90/904311/Grd_open/2'>GRD</scene> active site. Linked structurally to both HEAT ARM’s, it consists of mainly loops and helices. There is one single GRD binding site per protomer. In the closed state, Ras cannot bind the GRD site due to a steric hindrance in which Ras clashes with the N-HEAT ARM. Within the GRD site, the critical active site residue is Arg1276.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | Neurofibromin’s main catalytic domain is the <scene name='90/904311/Grd_open/2'>GRD</scene> active site. Linked structurally to both HEAT ARM’s, it consists of mainly loops and helices. There is one single GRD binding site per protomer. In the closed state, Ras cannot bind the GRD site due to a steric hindrance in which Ras clashes with the N-HEAT ARM. Within the GRD site, the critical active site residue is Arg1276.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
====GAPex==== | ====GAPex==== | ||
The <scene name='90/904311/Gapex_open/2'>GAPex subdomain</scene> of the GRD site lies between the Sec14-PH and GRD catalytic sites. This domain is non-catalytic and structurally consists of various loops and helices. Its main function is to bind SPRED-1. SPRED-1 is a recruiter protein that binds to this subdomain in the cytosol and recruits Neurofibromin to the plasma membrane. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | The <scene name='90/904311/Gapex_open/2'>GAPex subdomain</scene> of the GRD site lies between the Sec14-PH and GRD catalytic sites. This domain is non-catalytic and structurally consists of various loops and helices. Its main function is to bind SPRED-1. SPRED-1 is a recruiter protein that binds to this subdomain in the cytosol and recruits Neurofibromin to the plasma membrane. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
====Sec14-PH==== | ====Sec14-PH==== | ||
The <scene name='90/904311/Sec14ph_open/2'>Sec14-PH domain </scene> is linked and extends out from the HEAT ARM’s. Sec14-PH functions as a membrane associated domain and using a hydrophobic cavity binds to neurofibromin the plasma membrane. The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> also blocks Sec14-PH binding to the cell membrane due to blocking by GRD. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | The <scene name='90/904311/Sec14ph_open/2'>Sec14-PH domain </scene> is linked and extends out from the HEAT ARM’s. Sec14-PH functions as a membrane associated domain and using a hydrophobic cavity binds to neurofibromin the plasma membrane. The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> also blocks Sec14-PH binding to the cell membrane due to blocking by GRD. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
===Conformations=== | ===Conformations=== | ||
| + | |||
====Closed conformation==== | ====Closed conformation==== | ||
Ras is unable to bind to the GRD active site when both of the neurofibromin protomers are in the closed conformation. In the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>, one protomer has its domains shifted by a 130° rotation of three separate conformational change linkers. That rotation places <scene name='90/904311/Closed_arg/4'>Arg1276 in the closed conformation</scene> in an orientation that <scene name='90/904312/Closed_zoom/1'>sterically hinders the binding between Ras and Arg1276</scene> in the GRD site. Ras binding to the GRD site is inhibited by steric occlusion from the N-HEAT ARM. The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> can exist naturally without any form of stabilization but exists in a natural equilibrium with the <scene name='90/904311/Open_conformation/14'> open conformation</scene>.<ref name="Bourne"> DOI:10.1038/39470</ref> <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | Ras is unable to bind to the GRD active site when both of the neurofibromin protomers are in the closed conformation. In the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>, one protomer has its domains shifted by a 130° rotation of three separate conformational change linkers. That rotation places <scene name='90/904311/Closed_arg/4'>Arg1276 in the closed conformation</scene> in an orientation that <scene name='90/904312/Closed_zoom/1'>sterically hinders the binding between Ras and Arg1276</scene> in the GRD site. Ras binding to the GRD site is inhibited by steric occlusion from the N-HEAT ARM. The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> can exist naturally without any form of stabilization but exists in a natural equilibrium with the <scene name='90/904311/Open_conformation/14'> open conformation</scene>.<ref name="Bourne"> DOI:10.1038/39470</ref> <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
=====Zinc Stabilized===== | =====Zinc Stabilized===== | ||
The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> is stabilized by a zinc ion that prevents the shift back to the <scene name='90/904311/Open_conformation/14'> open conformation</scene>. Zinc binding is chelated by three residues (<scene name='90/904311/Zinc_binding_site/11'>Cys1032, His1558, His1576</scene>). These three residues are contributed by the N-HEAT domain (Cys1032) and the GAPex-subdomain (His1558 and His1576). Zinc stabilization keeps Neurofibromin in the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>, inhibiting Ras binding.<ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | The <scene name='90/904311/Closed_conformation/8'>closed conformation</scene> is stabilized by a zinc ion that prevents the shift back to the <scene name='90/904311/Open_conformation/14'> open conformation</scene>. Zinc binding is chelated by three residues (<scene name='90/904311/Zinc_binding_site/11'>Cys1032, His1558, His1576</scene>). These three residues are contributed by the N-HEAT domain (Cys1032) and the GAPex-subdomain (His1558 and His1576). Zinc stabilization keeps Neurofibromin in the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>, inhibiting Ras binding.<ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
====Open conformation==== | ====Open conformation==== | ||
In the <scene name='90/904311/Open_conformation/14'> open conformation</scene>, one protomer is shifted to allow Ras binding, while the other protomer remains in the closed conformation. In the <scene name='90/904311/Open_conformation/14'> open conformation</scene> one protomer is rotated 90°, facilitating binding between RAS and <scene name='90/904311/Arg_1276_open/1'>Arg1276</scene> | In the <scene name='90/904311/Open_conformation/14'> open conformation</scene>, one protomer is shifted to allow Ras binding, while the other protomer remains in the closed conformation. In the <scene name='90/904311/Open_conformation/14'> open conformation</scene> one protomer is rotated 90°, facilitating binding between RAS and <scene name='90/904311/Arg_1276_open/1'>Arg1276</scene> | ||
in the GRD site. To allow Ras binding, the GRD and Sec14-PH domains are reoriented away from one another and the GRD site is accessible for Ras binding. This conformational change to the open conformation is driven by rearrangement of three separate linkers (L1, L2, L3).<ref name="Bourne"> DOI:10.1038/39470</ref> <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | in the GRD site. To allow Ras binding, the GRD and Sec14-PH domains are reoriented away from one another and the GRD site is accessible for Ras binding. This conformational change to the open conformation is driven by rearrangement of three separate linkers (L1, L2, L3).<ref name="Bourne"> DOI:10.1038/39470</ref> <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
====Conformational Change Linkers==== | ====Conformational Change Linkers==== | ||
These three helical linkers (L1, L2, and L3)(<scene name='90/904311/Linker_closed/4'>Linkers in Closed Conformation</scene> | These three helical linkers (L1, L2, and L3)(<scene name='90/904311/Linker_closed/4'>Linkers in Closed Conformation</scene> | ||
| Line 49: | Line 60: | ||
<scene name='90/904312/Linker_closed/2'>(zoomed in)'</scene> and <scene name='90/904311/Linkers_open/1'>open conformation</scene> | <scene name='90/904312/Linker_closed/2'>(zoomed in)'</scene> and <scene name='90/904311/Linkers_open/1'>open conformation</scene> | ||
<scene name='90/904312/Zoomed_lo/1'>(zoomed in)'</scene>.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | <scene name='90/904312/Zoomed_lo/1'>(zoomed in)'</scene>.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
==Ras Binding== | ==Ras Binding== | ||
Arg1276 is the critical residue within the GRD site for Ras activation. In the open conformation, the arginine finger binds to the backbone γ-carbon of Y32 in Ras to assist in eventual hydrolysis of GTP (<scene name='90/904311/Arg_1276_open/14'>Arg 1276</scene> | Arg1276 is the critical residue within the GRD site for Ras activation. In the open conformation, the arginine finger binds to the backbone γ-carbon of Y32 in Ras to assist in eventual hydrolysis of GTP (<scene name='90/904311/Arg_1276_open/14'>Arg 1276</scene> | ||
| Line 59: | Line 71: | ||
==SPRED 1== | ==SPRED 1== | ||
<scene name='90/904311/Spred1_w_grd/2'>SPRED 1</scene> is another peripheral protein that interacts with the GRD domain of Neurofibromin. SPRED 1 recruits Neurofibromin from the cytosol to the plasma membrane to interact with Ras. Binding of SPRED 1 can occur in either the open or closed conformation and causes a structural rearrangement of the GRD domain and GAPex subdomain that has yet to be structuralized.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | <scene name='90/904311/Spred1_w_grd/2'>SPRED 1</scene> is another peripheral protein that interacts with the GRD domain of Neurofibromin. SPRED 1 recruits Neurofibromin from the cytosol to the plasma membrane to interact with Ras. Binding of SPRED 1 can occur in either the open or closed conformation and causes a structural rearrangement of the GRD domain and GAPex subdomain that has yet to be structuralized.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| + | |||
==Clinical Relevance== | ==Clinical Relevance== | ||
[https://en.wikipedia.org/wiki/Germline_mutation Germline mutations] are common in NF1 and often cause genetic tumor syndrome through misregulation of the Ras signaling pathway. [https://en.wikipedia.org/wiki/Somatic_mutation Somatic mutations] among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, tumors develop along the deep epidermis layer of the skin. Understanding how mutations affect the structure and function of neurofibromin will allow for advancements in treatment. | [https://en.wikipedia.org/wiki/Germline_mutation Germline mutations] are common in NF1 and often cause genetic tumor syndrome through misregulation of the Ras signaling pathway. [https://en.wikipedia.org/wiki/Somatic_mutation Somatic mutations] among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, tumors develop along the deep epidermis layer of the skin. Understanding how mutations affect the structure and function of neurofibromin will allow for advancements in treatment. | ||
DOI:10.3390/cells9112365</ref><ref name="Kioro"> DOI:10.1038/labinvest.2016.142</ref><ref name="Sabatini"> DOI:10.1007/s11940-015-0355-4</ref> | DOI:10.3390/cells9112365</ref><ref name="Kioro"> DOI:10.1038/labinvest.2016.142</ref><ref name="Sabatini"> DOI:10.1007/s11940-015-0355-4</ref> | ||
| - | |||
==FINAL SCENES COPY AND PASTE== | ==FINAL SCENES COPY AND PASTE== | ||
Revision as of 02:09, 19 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Neurofibromin 1
| |||||||||||