We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1703
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
===Active State=== | ===Active State=== | ||
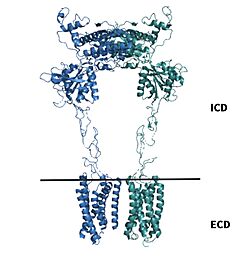
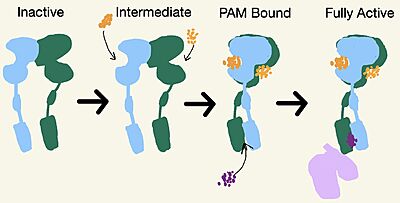
| - | Upon binding of the PAM, helix | + | Upon binding of the PAM, helix 6 is shifted downward in the TMD. This downward shift induces a reorientation of the TMD from its original TM3-TM4 asymmetric dimer interface in the inactive form to an <scene name='90/904308/Active_7_tm_transparent/1'>asymmetric TM6-TM6 interface</scene>. The downward shift of helix 6 is crucial for the receptor’s transformation from the inactive to the active form for 2 main reasons: (1) reorientation breaks key interactions in the TMD that stabilize the inactive form and (2) repositioning <scene name='90/904308/Active_structure/4'>intracellular loops</scene> of in the TMD to assist in the binding and recognitions of the <scene name='90/904308/G-protein/1'>G-Protein</scene>. The G-protein is made up of three subunits: <scene name='90/904308/Alpha_subunit/1'>α-subunit</scene>, <scene name='90/904308/Beta_subunit/1'>β-subunit</scene>, and a <scene name='90/904308/Gamma_subunit/1'>γ-subunit</scene>. |
====G-Protein Recognition==== | ====G-Protein Recognition==== | ||
| - | Transition to the active state also reorients helix 3 in both monomers to enable binding to the G-protein: Yet only one chain is required for full receptor activation. The intracellular region of helix 3 contributes the main interactions with the α-subunit of the G-protein. Intracellular Loop 2 also builds a polar interaction network with the G-protein through its ionic interactions with the <scene name='90/904308/Binding_recognition_site/2'> α-subunit</scene> of the G-protein. The ionic interactions formed further destabilize the inactive conformation<ref name="Lin"/>. | + | In order for the G-protein to bind to mGlu2, so that it can be fully active, the G-protein has to be recognized by the receptor. Transition to the active state also reorients helix 3 in both monomers to enable binding to the G-protein: Yet only one chain is required for full receptor activation. The intracellular region of helix 3 contributes the main interactions with the α-subunit of the G-protein. Intracellular Loop 2 also builds a polar interaction network with the G-protein through its ionic interactions with the <scene name='90/904308/Binding_recognition_site/2'> α-subunit</scene> of the G-protein. The ionic interactions formed further destabilize the inactive conformation<ref name="Lin"/>. |
====G-protein Binding==== | ====G-protein Binding==== | ||
Revision as of 02:47, 19 April 2022
Contents |
Metabotropic Glutamate Receptor 2
| |||||||||||
3D Structures
7mtq, mGlu2 inactive
7mtr, mGlu2 PAM bound
7mts, mGlu2 active
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ 2.0 2.1 2.2 2.3 Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3
- ↑ Du, Juan, et al. “Structures of Human mglu2 and mglu7 Homo- and Heterodimers.” Nature News, Nature Publishing Group, 16 June 2021, https://www.nature.com/articles/s41586-021-03641-w.>
- ↑ 4.0 4.1 “Metabotropic Glutamate Receptor.” Wikipedia, Wikimedia Foundation, 27 Mar. 2022, https://en.wikipedia.org/wiki/Metabotropic_glutamate_receptor
- ↑ \“Schizophrenia.” National Institute of Mental Health, U.S. Department of Health and Human Services, https://www.nimh.nih.gov/health/topics/schizophrenia
- ↑ 6.0 6.1 Ellaithy A, Younkin J, Gonzalez-Maeso J, Logothetis DE. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015 Aug;38(8):506-16. doi: 10.1016/j.tins.2015.06.002. Epub, 2015 Jul 4. PMID:26148747 doi:http://dx.doi.org/10.1016/j.tins.2015.06.002
- ↑ 7.0 7.1 7.2 7.3 Muguruza C, Meana JJ, Callado LF. Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front Pharmacol. 2016 May 20;7:130. doi: 10.3389/fphar.2016.00130. eCollection, 2016. PMID:27242534 doi:http://dx.doi.org/10.3389/fphar.2016.00130
Student Contributors
Frannie Brewer Ashley Wilkinson